BIS targeting induces cellular senescence through the regulation of 14-3-3 zeta/STAT3/SKP2/p27 in glioblastoma cells
- PMID: 25412315
- PMCID: PMC4260756
- DOI: 10.1038/cddis.2014.501
BIS targeting induces cellular senescence through the regulation of 14-3-3 zeta/STAT3/SKP2/p27 in glioblastoma cells
Abstract
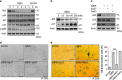
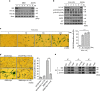
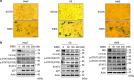
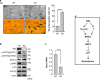
Cellular senescence is an important mechanism for preventing tumor progression. The elevated expression of Bcl-2-interacting cell death suppressor (BIS), an anti-apoptotic and anti-stress protein, often correlates with poor prognosis in several cancers including glioblastoma; however, the role of BIS in the regulation of senescence has not been well defined. Here, we describe for the first time that the depletion of BIS induces G1 arrest and cellular senescence through the accumulation of p27 that is independent of p53, p21 or p16. The increase in p27 expression in BIS-depleted cells was attributable to an impairment of the ubiquitin-mediated degradation of p27, which was caused by a decrease in S-phase kinase-associated protein 2 (SKP2) at the transcriptional level. As an underlying molecular mechanism, we demonstrate that the loss of activity of signal transducer and activator of transcription 3 (STAT3) was specifically linked to the suppression of SKP2 expression. Despite a reduction in phospho-STAT3 levels, total STAT3 levels were unexpectedly increased by BIS depletion, specifically in the insoluble fraction. Our results show that 14-3-3ζ expression is decreased by BIS knockdown and that 14-3-3ζ depletion per se significantly induced senescence phenotypes. In addition, the ectopic expression of 14-3-3ζ blocked senescence caused by BIS depletion, which was paralleled with a decrease in insoluble STAT3 in A172 glioblastoma cells. These findings indicate that the impairment of the protein quality control conferred by BIS and/or 14-3-3ζ is critical for BIS depletion-induced senescence. Moreover, BIS knockdown also induced senescence along with an accumulation of total STAT3 and p27 in several different cell types as well as embryonic fibroblasts derived from Bis-knock out mice with/without variations in 14-3-3ζ levels. Therefore, our findings suggest that a downregulation of BIS expression could serve as a potential strategy for restricting tumor progression via an induction of senescence through the regulation of STAT3/SKP2/p27 pathway.
Figures




References
-
- Schmitt CA. Senescence, apoptosis and therapy—cutting the lifelines of cancer. Nat Rev Cancer. 2003;3:286–295. - PubMed
-
- Nardella C, Clohessy JG, Alimonti A, Pandolfi PP. Pro-senescence therapy for cancer treatment. Nat Rev Cancer. 2011;11:503–511. - PubMed
-
- Kim WY, Sharpless NE. The regulation of INK4/ARF in cancer and aging. Cell. 2006;127:265–275. - PubMed
-
- Papazoglu C, Mills AA. p53: at the crossroad between cancer and ageing. J Pathol. 2007;211:124–133. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

