Human cytomegalovirus vaccine based on the envelope gH/gL pentamer complex
- PMID: 25412505
- PMCID: PMC4239111
- DOI: 10.1371/journal.ppat.1004524
Human cytomegalovirus vaccine based on the envelope gH/gL pentamer complex
Abstract
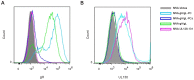
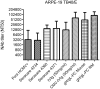
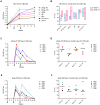
Human Cytomegalovirus (HCMV) utilizes two different pathways for host cell entry. HCMV entry into fibroblasts requires glycoproteins gB and gH/gL, whereas HCMV entry into epithelial and endothelial cells (EC) requires an additional complex composed of gH, gL, UL128, UL130, and UL131A, referred to as the gH/gL-pentamer complex (gH/gL-PC). While there are no established correlates of protection against HCMV, antibodies are thought to be important in controlling infection. Neutralizing antibodies (NAb) that prevent gH/gL-PC mediated entry into EC are candidates to be assessed for in vivo protective function. However, these potent NAb are predominantly directed against conformational epitopes derived from the assembled gH/gL-PC. To address these concerns, we constructed Modified Vaccinia Ankara (MVA) viruses co-expressing all five gH/gL-PC subunits (MVA-gH/gL-PC), subsets of gH/gL-PC subunits (gH/gL or UL128/UL130/UL131A), or the gB subunit from HCMV strain TB40/E. We provide evidence for cell surface expression and assembly of complexes expressing full-length gH or gB, or their secretion when the corresponding transmembrane domains are deleted. Mice or rhesus macaques (RM) were vaccinated three times with MVA recombinants and serum NAb titers that prevented 50% infection of human EC or fibroblasts by HCMV TB40/E were determined. NAb responses induced by MVA-gH/gL-PC blocked HCMV infection of EC with potencies that were two orders of magnitude greater than those induced by MVA expressing gH/gL, UL128-UL131A, or gB. In addition, MVA-gH/gL-PC induced NAb responses that were durable and efficacious to prevent HCMV infection of Hofbauer macrophages, a fetal-derived cell localized within the placenta. NAb were also detectable in saliva of vaccinated RM and reached serum peak levels comparable to NAb titers found in HCMV hyperimmune globulins. This vaccine based on a translational poxvirus platform co-delivers all five HCMV gH/gL-PC subunits to achieve robust humoral responses that neutralize HCMV infection of EC, placental macrophages and fibroblasts, properties of potential value in a prophylactic vaccine.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







References
-
- Plotkin SA (1999) Vaccination against cytomegalovirus, the changeling demon. Pediatr Infect Dis J 18: 313–325. - PubMed
-
- Weller TH (1971) The cytomegaloviruses: ubiquitous agents with protean clinical manifestations. II. N Engl J Med 285: 267–274. - PubMed
-
- Pereira L, Maidji E (2008) Cytomegalovirus infection in the human placenta: maternal immunity and developmentally regulated receptors on trophoblasts converge. Curr Top Microbiol Immunol 325: 383–395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 AI050409/AI/NIAID NIH HHS/United States
- R01 AI103960/AI/NIAID NIH HHS/United States
- P51 OD011107/OD/NIH HHS/United States
- R01 CA077544/CA/NCI NIH HHS/United States
- AI103960/AI/NIAID NIH HHS/United States
- R01 AI063356/AI/NIAID NIH HHS/United States
- P51 RR000169/RR/NCRR NIH HHS/United States
- AI63356/AI/NIAID NIH HHS/United States
- P30 CA033572/CA/NCI NIH HHS/United States
- CA077544/CA/NCI NIH HHS/United States
- P01 CA030206/CA/NCI NIH HHS/United States
- CA030206/CA/NCI NIH HHS/United States
- P51 RR00169/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

