p62 Deficiency Enhances α-Synuclein Pathology in Mice
- PMID: 25412696
- PMCID: PMC8029467
- DOI: 10.1111/bpa.12214
p62 Deficiency Enhances α-Synuclein Pathology in Mice
Abstract
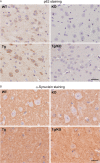
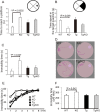
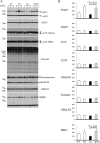
In Lewy body disease (LBD) such as dementia with LBs and Parkinson's disease, several lines of evidence show that disrupted proteolysis occurs. p62/SQSTM1 (p62) is highly involved with intracellular proteolysis and is a component of ubiquitin-positive inclusions in various neurodegenerative disorders. However, it is not clear whether p62 deficiency affects inclusion formation and abnormal protein accumulation. To answer this question, we used a mouse model of LBD that lacks p62, and found that LB-like inclusions were observed in transgenic mice that overexpressed α-synuclein (Tg mice) with or without the p62 protein. p62 deficiency enhanced α-synuclein pathology with regard to the number of inclusions and staining intensity compared with Tg mice that expressed p62. To further investigate the molecular mechanisms associated with the loss of p62 in Tg mice, we assessed the mRNA and protein levels of several molecules, and found that the neighbor of the brca1 gene (NBr1), which is functionally and structurally similar to p62, is increased in Tg mice without p62 compared with control Tg mice. These findings suggest that p62 and NBR1 affect the pathogenesis of neurodegenerative diseases through the cooperative modulation of α-synuclein aggregation.
Keywords: Lewy body disease; Parkinson's disease; p62/Sequestsome 1/SQSTM1; proteolysis; stress; α-synuclein.
© 2014 International Society of Neuropathology.
Figures




References
-
- Braak H, Thal DR, Del Tredici K (2011) Nerve cells immunoreactive for p62 in select hypothalamic and brainstem nuclei of controls and Parkinson's disease cases. J Neural Transm 118:809–819. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

