Nitric oxide regulates synaptic transmission between spiny projection neurons
- PMID: 25413364
- PMCID: PMC4267338
- DOI: 10.1073/pnas.1420162111
Nitric oxide regulates synaptic transmission between spiny projection neurons
Erratum in
-
Correction.Proc Natl Acad Sci U S A. 2015 Apr 7;112(14):E1811. doi: 10.1073/pnas.1504794112. Epub 2015 Mar 18. Proc Natl Acad Sci U S A. 2015. PMID: 25787251 Free PMC article. No abstract available.
Abstract
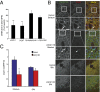
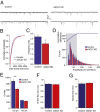
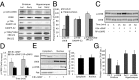
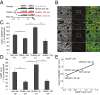
Recurrent axon collaterals are a major means of communication between spiny projection neurons (SPNs) in the striatum and profoundly affect the function of the basal ganglia. However, little is known about the molecular and cellular mechanisms that underlie this communication. We show that intrastriatal nitric oxide (NO) signaling elevates the expression of the vesicular GABA transporter (VGAT) within recurrent collaterals of SPNs. Down-regulation of striatal NO signaling resulted in an attenuation of GABAergic signaling in SPN local collaterals, down-regulation of VGAT expression in local processes of SPNs, and impaired motor behavior. PKG1 and cAMP response element-binding protein are involved in the signal transduction that transcriptionally regulates VGAT by NO. These data suggest that transcriptional control of the vesicular GABA transporter by NO regulates GABA transmission and action selection.
Keywords: BacTRAP; axon collaterals; guanylyl cyclase; spiny projecting neurons; vesicular GABA transporter.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
When inhibition goes incognito: feedback interaction between spiny projection neurons in striatal function.Trends Neurosci. 2003 Aug;26(8):436-43. doi: 10.1016/S0166-2236(03)00196-6. Trends Neurosci. 2003. PMID: 12900175 Review.
-
Fluorescent labeling of both GABAergic and glycinergic neurons in vesicular GABA transporter (VGAT)-venus transgenic mouse.Neuroscience. 2009 Dec 15;164(3):1031-43. doi: 10.1016/j.neuroscience.2009.09.010. Epub 2009 Sep 17. Neuroscience. 2009. PMID: 19766173
-
Co-localization of GABA with other neuroactive substances in the basal ganglia.Prog Brain Res. 2007;160:273-84. doi: 10.1016/S0079-6123(06)60016-2. Prog Brain Res. 2007. PMID: 17499120 Review.
-
gamma-Aminobutyric acid (GABA) signaling components in Drosophila: immunocytochemical localization of GABA(B) receptors in relation to the GABA(A) receptor subunit RDL and a vesicular GABA transporter.J Comp Neurol. 2007 Nov 1;505(1):18-31. doi: 10.1002/cne.21472. J Comp Neurol. 2007. PMID: 17729251
-
Embryonic development of GABAergic signaling in the mouse spinal trigeminal nucleus interpolaris.Neurosci Lett. 2014 Apr 30;566:221-5. doi: 10.1016/j.neulet.2014.02.057. Epub 2014 Mar 7. Neurosci Lett. 2014. PMID: 24607929
Cited by
-
The dependence of neuronal encoding efficiency on Hebbian plasticity and homeostatic regulation of neurotransmitter release.Front Cell Neurosci. 2015 Apr 28;9:164. doi: 10.3389/fncel.2015.00164. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25972786 Free PMC article.
-
Dopaminergic modulation of striatal function and Parkinson's disease.J Neural Transm (Vienna). 2019 Apr;126(4):411-422. doi: 10.1007/s00702-019-01997-y. Epub 2019 Apr 1. J Neural Transm (Vienna). 2019. PMID: 30937538 Free PMC article. Review.
-
Xylazole inhibits NO-cGMP pathway in fetal rat nerve cells.J Vet Sci. 2022 Jan;23(1):e16. doi: 10.4142/jvs.21188. J Vet Sci. 2022. PMID: 35088953 Free PMC article.
-
TGF-β/Smad3 Signalling Modulates GABA Neurotransmission: Implications in Parkinson's Disease.Int J Mol Sci. 2020 Jan 16;21(2):590. doi: 10.3390/ijms21020590. Int J Mol Sci. 2020. PMID: 31963327 Free PMC article. Review.
-
Interneuronal Nitric Oxide Signaling Mediates Post-synaptic Long-Term Depression of Striatal Glutamatergic Synapses.Cell Rep. 2015 Nov 17;13(7):1336-1342. doi: 10.1016/j.celrep.2015.10.015. Epub 2015 Nov 5. Cell Rep. 2015. PMID: 26549446 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

