Comparative in vitro and in vivo pharmacological investigation of platinum(IV) complexes as novel anticancer drug candidates for oral application
- PMID: 25413442
- PMCID: PMC4351919
- DOI: 10.1007/s00775-014-1214-6
Comparative in vitro and in vivo pharmacological investigation of platinum(IV) complexes as novel anticancer drug candidates for oral application
Abstract
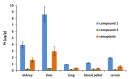
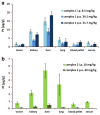
Platinum(IV) complexes are promising candidates as prodrugs for oral application in anticancer chemotherapy. However, only a few Pt(IV) compounds entered (pre)clinical trials, e.g. satraplatin, while most of the others were only tested in vitro. Aim of the study was investigation of the in vivo pharmacological behavior as well as the anticancer activity of two novel platinum(IV) complexes vs. satraplatin. The drugs were selected due to significantly different in vitro cytotoxicity while sharing some physicochemical properties (e.g. lipophilicity). Initial experiments indicated that the highly in vitro cytotoxic compound 1 ((OC-6-33)-dichloridobis((4-ethoxy)-4-oxobutanoato)-bis(ethylamine)platinum(IV)) was also characterized by high drug absorption and tissue platinum levels after oral application. Interestingly, analysis of serum samples using SEC-ICP-MS revealed that the administered drugs have completely been metabolized and/or bound to proteins in serum within 2 h after treatment. With regard to the activity in vivo, the outcomes were rather unexpected: although potent anticancer effect of 1 was observed in cell culture, the effects in vivo were rather minor. Nevertheless, 1 was superior to 2 ((OC-6-33)-diammine(cyclobutane-1,1-dicarboxylato)-bis((4-cyclopentylamino)-4-oxobutanoato)platinum(IV)) after i.p. administration, which was, at least to some extent, in accordance to the cell culture experiments. After oral gavage, both compounds exhibited comparable activity. This is remarkable considering the distinctly lower activity of 2 in cell culture as well as the low platinum levels detected both in serum and tissues after oral application. Consequently, our data indicate that the prediction of in vivo anticancer activity by cell culture experiments is not trivial, especially for orally applied drugs.
Figures


References
-
- Wheate NJ, Walker S, Craig GE, Oun R. Dalton Trans. 2010;39:8113–8127. - PubMed
-
- Yao X, Panichpisal K, Kurtzman N, Kenneth N. Am J Med Sci. 2007;334:115–124. - PubMed
-
- Heffeter P, Jungwirth U, Jakupec M, Hartinger C, Galanski M, Elbling L, Micksche M, Keppler B, Berger W. Drug Resist Updat. 2008;11:1–16. - PubMed
-
- Kelland LR. Nat Rev Cancer. 2007;7:573–584. - PubMed
-
- Jakupec MA, Galanski M, Arion VB, Hartinger CG, Keppler BK. Dalton Trans. 2008;2:183–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

