Wordless intervention for epilepsy in learning disabilities (WIELD): study protocol for a randomized controlled feasibility trial
- PMID: 25414095
- PMCID: PMC4289382
- DOI: 10.1186/1745-6215-15-455
Wordless intervention for epilepsy in learning disabilities (WIELD): study protocol for a randomized controlled feasibility trial
Abstract
Background: Epilepsy is the most common neurological problem that affects people with learning disabilities. The high seizure frequency, resistance to treatments, associated skills deficit and co-morbidities make the management of epilepsy particularly challenging for people with learning disabilities. The Books Beyond Words booklet for epilepsy uses images to help people with learning disabilities manage their condition and improve quality of life. Our aim is to conduct a randomized controlled feasibility trial exploring key methodological, design and acceptability issues, in order to subsequently undertake a large-scale randomized controlled trial of the Books Beyond Words booklet for epilepsy.
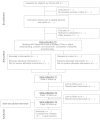
Methods/design: We will use a two-arm, single-centre randomized controlled feasibility design, over a 20-month period, across five epilepsy clinics in Hertfordshire, United Kingdom. We will recruit 40 eligible adults with learning disabilities and a confirmed diagnosis of epilepsy and will randomize them to use either the Books Beyond Words booklet plus usual care (intervention group) or to receive routine information and services (control group). We will collect quantitative data about the number of eligible participants, number of recruited participants, demographic data, discontinuation rates, variability of the primary outcome measure (quality of life: Epilepsy and Learning Disabilities Quality of Life scale), seizure severity, seizure control, intervention's patterns of use, use of other epilepsy-related information, resource use and the EQ-5D-5L health questionnaire. We will also gather qualitative data about the feasibility and acceptability of the study procedures and the Books Beyond Words booklet. Ethical approval for this study was granted on 28 April 2014, by the Wales Research Ethics Committee 5. Recruitment began on 1 July 2014.
Discussion: The outcomes of this feasibility study will be used to inform the design and methodology of a definitive study, adequately powered to determine the impact of the Books Beyond Words intervention to improve the management of epilepsy in people with learning disabilities.
Trial registration: http://ISRCTN80067039 (Date of ISRCTN assignation: 23 April 2014).
Figures
Similar articles
-
Wordless intervention for people with epilepsy and learning disabilities (WIELD): a randomised controlled feasibility trial.BMJ Open. 2016 Nov 10;6(11):e012993. doi: 10.1136/bmjopen-2016-012993. BMJ Open. 2016. PMID: 28186943 Free PMC article. Clinical Trial.
-
Faecal incontinence intervention study (FINS): self-management booklet information with or without nurse support to improve continence in people with inflammatory bowel disease: study protocol for a randomized controlled trial.Trials. 2015 Oct 6;16:444. doi: 10.1186/s13063-015-0962-0. Trials. 2015. PMID: 26445224 Free PMC article. Clinical Trial.
-
Emotional literacy programme in special schools for children with a learning disability in England: the ZF-SEND feasibility RCT.Public Health Res (Southampt). 2024 Dec;12(15):1-105. doi: 10.3310/JTJY8001. Public Health Res (Southampt). 2024. PMID: 39641743 Clinical Trial.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Seizure first aid training for people with epilepsy attending emergency departments and their significant others: the SAFE intervention and feasibility RCT.Southampton (UK): NIHR Journals Library; 2020 Oct. Southampton (UK): NIHR Journals Library; 2020 Oct. PMID: 33112533 Free Books & Documents. Review.
Cited by
-
Wordless intervention for people with epilepsy and learning disabilities (WIELD): a randomised controlled feasibility trial.BMJ Open. 2016 Nov 10;6(11):e012993. doi: 10.1136/bmjopen-2016-012993. BMJ Open. 2016. PMID: 28186943 Free PMC article. Clinical Trial.
-
"Sometimes, it just stops me from doing anything": A qualitative exploration of epilepsy management in people with intellectual disabilities and their carers.Epilepsy Behav. 2016 Nov;64(Pt A):133-139. doi: 10.1016/j.yebeh.2016.09.029. Epub 2016 Oct 11. Epilepsy Behav. 2016. PMID: 27736660 Free PMC article.
-
A systematic review and narrative synthesis of group self-management interventions for adults with epilepsy.BMC Neurol. 2017 Jun 17;17(1):114. doi: 10.1186/s12883-017-0893-3. BMC Neurol. 2017. PMID: 28623909 Free PMC article.
References
-
- Mariani E, Ferini-Strambi L, Sala M, Erminio C, Smirne S. Epilepsy in institutionalized patients with encephalopathy: clinical aspects and nosological considerations. Am J Ment Retard. 1993;98(Suppl):27–33. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous