Green tea extract attenuates muscle loss and improves muscle function during disuse, but fails to improve muscle recovery following unloading in aged rats
- PMID: 25414242
- PMCID: PMC4312851
- DOI: 10.1152/japplphysiol.00674.2014
Green tea extract attenuates muscle loss and improves muscle function during disuse, but fails to improve muscle recovery following unloading in aged rats
Abstract
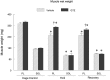
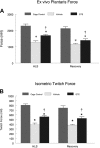
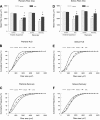
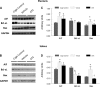
In this study we tested the hypothesis that green tea extract (GTE) would improve muscle recovery after reloading following disuse. Aged (32 mo) Fischer 344 Brown Norway rats were randomly assigned to receive either 14 days of hindlimb suspension (HLS) or 14 days of HLS followed by normal ambulatory function for 14 days (recovery). Additional animals served as cage controls. The rats were given GTE (50 mg/kg body wt) or water (vehicle) by gavage 7 days before and throughout the experimental periods. Compared with vehicle treatment, GTE significantly attenuated the loss of hindlimb plantaris muscle mass (-24.8% vs. -10.7%, P < 0.05) and tetanic force (-43.7% vs. -25.9%, P <0.05) during HLS. Although GTE failed to further improve recovery of muscle function or mass compared with vehicle treatment, animals given green tea via gavage maintained the lower losses of muscle mass that were found during HLS (-25.2% vs. -16.0%, P < 0.05) and force (-45.7 vs. -34.4%, P < 0.05) after the reloading periods. In addition, compared with vehicle treatment, GTE attenuated muscle fiber cross-sectional area loss in both plantaris (-39.9% vs. -23.9%, P < 0.05) and soleus (-37.2% vs. -17.6%) muscles after HLS. This green tea-induced difference was not transient but was maintained over the reloading period for plantaris (-45.6% vs. -21.5%, P <0.05) and soleus muscle fiber cross-sectional area (-38.7% vs. -10.9%, P <0.05). GTE increased satellite cell proliferation and differentiation in plantaris and soleus muscles during recovery from HLS compared with vehicle-treated muscles and decreased oxidative stress and abundance of the Bcl-2-associated X protein (Bax), yet this did not further improve muscle recovery in reloaded muscles. These data suggest that muscle recovery following disuse in aging is complex. Although satellite cell proliferation and differentiation are critical for muscle repair to occur, green tea-induced changes in satellite cell number is by itself insufficient to improve muscle recovery following a period of atrophy in old rats.
Keywords: EGCg; apoptosis; catechin; disuse; exercise; muscle atrophy; reloading; sarcopenia.
Copyright © 2015 the American Physiological Society.
Figures





References
-
- Adams GR. Satellite cell proliferation and skeletal muscle hypertrophy. Appl Physiol Nutr Metab 31: 782–790, 2006. - PubMed
-
- Adams GR, Caiozzo VJ, Haddad F, Baldwin KM. Cellular and molecular responses to increased skeletal muscle loading after irradiation. Am J Physiol Cell Physiol 283: C1182–C1195, 2002. - PubMed
-
- Allen DL, Linderman JK, Roy RR, Bigbee AJ, Grindeland RE, Mukku V, Edgerton VR. Apoptosis: a mechanism contributing to remodeling of skeletal muscle in response to hindlimb unweighting. Am J Physiol Cell Physiol 273: C579–C587, 1997. - PubMed
-
- Alway SE. Attenuation of Ca(2+)-activated ATPase and shortening velocity in hypertrophied fast twitch skeletal muscle from aged Japanese quail. Exp Gerontol 37: 665–678, 2002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

