Astrocyte development and heterogeneity
- PMID: 25414368
- PMCID: PMC4292163
- DOI: 10.1101/cshperspect.a020362
Astrocyte development and heterogeneity
Abstract
Astrocytes have many roles within the brain parenchyma, and a subpopulation restricted to germinal niches functions as neural stem cells (NSCs) that produce various types of neuronal progeny in relation to spatiotemporal factors. A growing body of evidence supports the concept of morphological and molecular differences between astrocytes in different brain regions, which might relate to their derivation from regionally patterned radial glia. Indeed, the notion that astrocytes are molecularly and functionally heterogeneous could help explain how the central nervous system (CNS) retains embryonic positional information into adulthood. Here, we discuss recent evidence for regionally encoded functions of astrocytes in the developing and adult CNS to provide an integrated concept of the origin and possible function of astrocyte heterogeneity. We focus on the regionalization of NSCs in the ventricular-subventricular zone (V-SVZ) of the adult mammalian brain and emerging evidence for a segmental organization of astrocytes in the developing spinal cord and forebrain. We propose that astrocytes' diversity will provide fundamental clues to understand regional brain organization and function.
Copyright © 2015 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
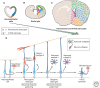

References
-
- Allen ZJ II, Waclaw RR, Colbert MC, Campbell K 2007. Molecular identity of olfactory bulb interneurons: Transcriptional codes of periglomerular neuron subtypes. J Mol Histol 38: 517–525. - PubMed
-
- Alvarez-Buylla A, García-Verdugo JM, Tramontin AD 2001. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci 2: 287–293. - PubMed
-
- Alvarez-Buylla A, Kohwi M, Nguyen TM, Merkle FT 2008. The heterogeneity of adult neural stem cells and the emerging complexity of their niche. Cold Spring Harb Symp Quant Biol 73: 357–365. - PubMed
-
- Anthony TE, Klein C, Fishell G, Heintz N 2004. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron 41: 881–890. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
