RUNX1, a transcription factor mutated in breast cancer, controls the fate of ER-positive mammary luminal cells
- PMID: 25415051
- PMCID: PMC4381933
- DOI: 10.7554/eLife.03881
RUNX1, a transcription factor mutated in breast cancer, controls the fate of ER-positive mammary luminal cells
Abstract
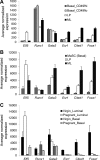
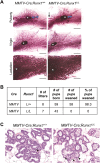
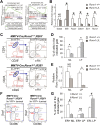
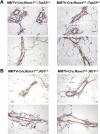
RUNX1 encodes a RUNX family transcription factor (TF) and was recently identified as a novel mutated gene in human luminal breast cancers. We found that Runx1 is expressed in all subpopulations of murine mammary epithelial cells (MECs) except the secretory alveolar luminal cells. Conditional knockout of Runx1 in MECs by MMTV-Cre led to a decrease in luminal MECs, largely due to a profound reduction in the estrogen receptor (ER)-positive mature luminal subpopulation, a phenotype that could be rescued by the loss of either Trp53 or Rb1. Mechanistically RUNX1 represses Elf5, a master regulatory TF gene for alveolar cells, and regulates mature luminal TF/co-factor genes (e.g., Foxa1 and Cited1) involved in the ER program. Collectively, our data identified a key regulator of the ER⁺ luminal lineage whose disruption may contribute to the development of ER⁺ luminal breast cancer when under the background of either TP53 or RB1 loss.
Keywords: ELF5; RUNX1; cell fate; developmental biology; estrogen receptor; human; human biology; luminal breast cancer; master regulatory transcription factor; medicine; mouse; stem cells.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures






Similar articles
-
Tip30 controls differentiation of murine mammary luminal progenitor to estrogen receptor-positive luminal cell through regulating FoxA1 expression.Cell Death Dis. 2014 May 22;5(5):e1242. doi: 10.1038/cddis.2014.224. Cell Death Dis. 2014. PMID: 24853420 Free PMC article.
-
Identification of cellular and genetic drivers of breast cancer heterogeneity in genetically engineered mouse tumour models.J Pathol. 2014 Jun;233(2):124-37. doi: 10.1002/path.4345. J Pathol. 2014. PMID: 24615332
-
Loss of BRCA1 leads to an increase in epidermal growth factor receptor expression in mammary epithelial cells, and epidermal growth factor receptor inhibition prevents estrogen receptor-negative cancers in BRCA1-mutant mice.Breast Cancer Res. 2011 Mar 11;13(2):R30. doi: 10.1186/bcr2850. Breast Cancer Res. 2011. PMID: 21396117 Free PMC article.
-
Mechanisms of Regulation of Cell Fate in Breast Development and Cancer.Adv Exp Med Biol. 2025;1464:167-184. doi: 10.1007/978-3-031-70875-6_10. Adv Exp Med Biol. 2025. PMID: 39821026 Review.
-
Hormone-sensing mammary epithelial progenitors: emerging identity and hormonal regulation.J Mammary Gland Biol Neoplasia. 2015 Jun;20(1-2):75-91. doi: 10.1007/s10911-015-9344-1. Epub 2015 Sep 21. J Mammary Gland Biol Neoplasia. 2015. PMID: 26390871 Review.
Cited by
-
Modeling Breast Cancer via an Intraductal Injection of Cre-expressing Adenovirus into the Mouse Mammary Gland.J Vis Exp. 2019 Jun 7;(148):10.3791/59502. doi: 10.3791/59502. J Vis Exp. 2019. PMID: 31233030 Free PMC article.
-
Endocrine resistance in breast cancer: from molecular mechanisms to therapeutic strategies.J Mol Med (Berl). 2021 Dec;99(12):1691-1710. doi: 10.1007/s00109-021-02136-5. Epub 2021 Oct 8. J Mol Med (Berl). 2021. PMID: 34623477 Free PMC article. Review.
-
Profiling the epigenetic interplay of lncRNA RUNXOR and oncogenic RUNX1 in breast cancer cells by gene in situ cis-activation.Am J Cancer Res. 2019 Aug 1;9(8):1635-1649. eCollection 2019. Am J Cancer Res. 2019. PMID: 31497347 Free PMC article.
-
Suppression of Breast Cancer Stem Cells and Tumor Growth by the RUNX1 Transcription Factor.Mol Cancer Res. 2018 Dec;16(12):1952-1964. doi: 10.1158/1541-7786.MCR-18-0135. Epub 2018 Aug 6. Mol Cancer Res. 2018. PMID: 30082484 Free PMC article.
-
Master regulator genes and their impact on major diseases.PeerJ. 2020 Oct 6;8:e9952. doi: 10.7717/peerj.9952. eCollection 2020. PeerJ. 2020. PMID: 33083114 Free PMC article.
References
-
- Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, Hartley L, Robb L, Grosveld FG, van der Wees J, Lindeman GJ, Visvader JE. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nature Cell Biology. 2007;9:201–209. doi: 10.1038/ncb1530. - DOI - PubMed
-
- Asselin-Labat ML, Sutherland KD, Vaillant F, Gyorki DE, Wu D, Holroyd S, Breslin K, Ward T, Shi W, Bath ML, Deb S, Fox SB, Smyth GK, Lindeman GJ, Visvader JE. Gata-3 negatively regulates the tumor-initiating capacity of mammary luminal progenitor cells and targets the putative tumor suppressor caspase-14. Molecular and Cellular Biology. 2011;31:4609–4622. doi: 10.1128/MCB.05766-11. - DOI - PMC - PubMed
-
- Banerji S, Cibulskis K, Rangel-Escareno C, Brown KK, Carter SL, Frederick AM, Lawrence MS, Sivachenko AY, Sougnez C, Zou L, Cortes ML, Fernandez-Lopez JC, Peng S, Ardlie KG, Auclair D, Bautista-Piña V, Duke F, Francis J, Jung J, Maffuz-Aziz A, Onofrio RC, Parkin M, Pho NH, Quintanar-Jurado V, Ramos AH, Rebollar-Vega R, Rodriguez-Cuevas S, Romero-Cordoba SL, Schumacher SE, Stransky N, Thompson KM, Uribe-Figueroa L, Baselga J, Beroukhim R, Polyak K, Sgroi DC, Richardson AL, Jimenez-Sanchez G, Lander ES, Gabriel SB, Garraway LA, Golub TR, Melendez-Zajgla J, Toker A, Getz G, Hidalgo-Miranda A, Meyerson M. Sequence analysis of mutations and translocations across breast cancer subtypes. Nature. 2012;486:405–409. doi: 10.1038/nature11154. - DOI - PMC - PubMed
-
- Bernardo GM, Lozada KL, Miedler JD, Harburg G, Hewitt SC, Mosley JD, Godwin AK, Korach KS, Visvader JE, Kaestner KH, Abdul-Karim FW, Montano MM, Keri RA. FOXA1 is an essential determinant of ERalpha expression and mammary ductal morphogenesis. Development. 2010;137:2045–2054. doi: 10.1242/dev.043299. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

