Fast retrieval and autonomous regulation of single spontaneously recycling synaptic vesicles
- PMID: 25415052
- PMCID: PMC4270043
- DOI: 10.7554/eLife.03658
Fast retrieval and autonomous regulation of single spontaneously recycling synaptic vesicles
Abstract
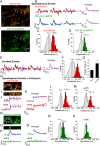
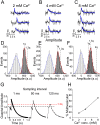
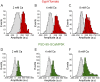
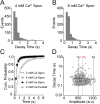
Presynaptic terminals release neurotransmitters spontaneously in a manner that can be regulated by Ca(2+). However, the mechanisms underlying this regulation are poorly understood because the inherent stochasticity and low probability of spontaneous fusion events has curtailed their visualization at individual release sites. Here, using pH-sensitive optical probes targeted to synaptic vesicles, we visualized single spontaneous fusion events and found that they are retrieved extremely rapidly with faster re-acidification kinetics than their action potential-evoked counterparts. These fusion events were coupled to postsynaptic NMDA receptor-driven Ca(2+) signals, and at elevated Ca(2+) concentrations there was an increase in the number of vesicles that would undergo fusion. Furthermore, spontaneous vesicle fusion propensity in a synapse was Ca(2+)-dependent but regulated autonomously: independent of evoked fusion probability at the same synapse. Taken together, these results expand classical quantal analysis to incorporate endocytic and exocytic phases of single fusion events and uncover autonomous regulation of spontaneous fusion.
Keywords: cell biology; mouse; neuroscience; rat; spontaneous neurotransmitter release; synaptic terminals; synaptic vesicle recycling.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures


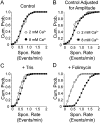
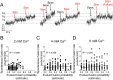

References
-
- Akerboom J, Chen TW, Wardill TJ, Tian L, Marvin JS, Mutlu S, Calderon NC, Esposti F, Borghuis BG, Sun XR, Gordus A, Orger MB, Portugues R, Engert F, Macklin JJ, Filosa A, Aggarwal A, Kerr RA, Takagi R, Kracun S, Shigetomi E, Khakh BS, Baier H, Lagnado L, Wang SS, Bargmann CI, Kimmel BE, Jayaraman V, Svoboda K, Kim DS, Schreiter ER, Looger LL. Optimization of a GCaMP calcium indicator for neural activity imaging. The Journal of Neuroscience. 2012;32:13819–13840. doi: 10.1523/JNEUROSCI.2601-12.2012. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

