Involvement of the kynurenine pathway in human glioma pathophysiology
- PMID: 25415278
- PMCID: PMC4240539
- DOI: 10.1371/journal.pone.0112945
Involvement of the kynurenine pathway in human glioma pathophysiology
Abstract
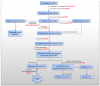
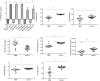
The kynurenine pathway (KP) is the principal route of L-tryptophan (TRP) catabolism leading to the production of kynurenine (KYN), the neuroprotectants, kynurenic acid (KYNA) and picolinic acid (PIC), the excitotoxin, quinolinic acid (QUIN) and the essential pyridine nucleotide, nicotinamide adenine dinucleotide (NAD(+)). The enzymes indoleamine 2,3-dioxygenase-1 (IDO-1), indoleamine 2,3-dioxygenase-2 (IDO-2) and tryptophan 2,3-dioxygenase (TDO-2) initiate the first step of the KP. IDO-1 and TDO-2 induction in tumors are crucial mechanisms implicated to play pivotal roles in suppressing anti-tumor immunity. Here, we report the first comprehensive characterisation of the KP in 1) cultured human glioma cells and 2) plasma from patients with glioblastoma (GBM). Our data revealed that interferon-gamma (IFN-γ) stimulation significantly potentiated the expression of the KP enzymes, IDO-1 IDO-2, kynureninase (KYNU), kynurenine hydroxylase (KMO) and significantly down-regulated 2-amino-3-carboxymuconate semialdehyde decarboxylase (ACMSD) and kynurenine aminotransferase-I (KAT-I) expression in cultured human glioma cells. This significantly increased KP activity but significantly lowered the KYNA/KYN neuroprotective ratio in human cultured glioma cells. KP activation (KYN/TRP) was significantly higher, whereas the concentrations of the neuroreactive KP metabolites TRP, KYNA, QUIN and PIC and the KYNA/KYN ratio were significantly lower in GBM patient plasma (n = 18) compared to controls. These results provide further evidence for the involvement of the KP in glioma pathophysiology and highlight a potential role of KP products as novel and highly attractive therapeutic targets to evaluate for the treatment of brain tumors, aimed at restoring anti-tumor immunity and reducing the capacity for malignant cells to produce NAD(+), which is necessary for energy production and DNA repair.
Conflict of interest statement
Figures



References
-
- Sreekanthreddy P, Srinivasan H, Kumar DM, Nijaguna MB, Sridevi S, et al. Identification of Potential Serum Biomarkers of Glioblastoma: Serum Osteopontin Levels Correlate with Poor Prognosis. Cancer Epidemiology Biomarkers & Prevention. 2010;19:1409–1422. - PubMed
-
- DeAngelis LM. Medical progress: Brain tumors. New England Journal of Medicine. 2001;344:114–123. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New England Journal of Medicine. 2005;352:987–996. - PubMed
-
- McLendon RE, Halperin EC. Is the long-term survival of patients with intracranial glioblastoma multiforme overstated? Cancer. 2003;98:1745–1748. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

