Viral-mediated RdCVF and RdCVFL expression protects cone and rod photoreceptors in retinal degeneration
- PMID: 25415434
- PMCID: PMC4382269
- DOI: 10.1172/JCI65654
Viral-mediated RdCVF and RdCVFL expression protects cone and rod photoreceptors in retinal degeneration
Abstract
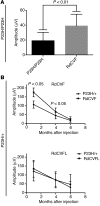
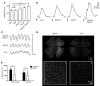
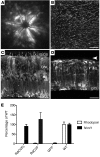
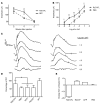
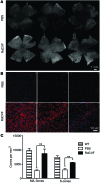
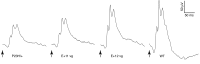
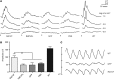
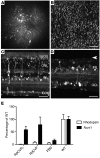
Alternative splicing of nucleoredoxin-like 1 (Nxnl1) results in 2 isoforms of the rod-derived cone viability factor. The truncated form (RdCVF) is a thioredoxin-like protein secreted by rods that promotes cone survival, while the full-length isoform (RdCVFL), which contains a thioredoxin fold, is involved in oxidative signaling and protection against hyperoxia. Here, we evaluated the effects of these different isoforms in 2 murine models of rod-cone dystrophy. We used adeno-associated virus (AAV) to express these isoforms in mice and found that both systemic and intravitreal injection of engineered AAV vectors resulted in RdCVF and RdCVFL expression in the eye. Systemic delivery of AAV92YF vectors in neonates resulted in earlier onset of RdCVF and RdCVFL expression compared with that observed with intraocular injection using the same vectors at P14. We also evaluated the efficacy of intravitreal injection using a recently developed photoreceptor-transducing AAV variant (7m8) at P14. Systemic administration of AAV92YF-RdCVF improved cone function and delayed cone loss, while AAV92YF-RdCVFL increased rhodopsin mRNA and reduced oxidative stress by-products. Intravitreal 7m8-RdCVF slowed the rate of cone cell death and increased the amplitude of the photopic electroretinogram. Together, these results indicate different functions for Nxnl1 isoforms in the retina and suggest that RdCVF gene therapy has potential for treating retinal degenerative disease.
Figures
References
-
- Daiger SP. RetNet: Summaries of Genes and Loci Causing Retinal Diseases. University of Texas Health Science Center Web site. https://sph.uth.edu/retnet/sum-dis.htm Updated October 14, 2014. Accessed October 28, 2014
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

