Survivin-specific T cell receptor targets tumor but not T cells
- PMID: 25415440
- PMCID: PMC4382259
- DOI: 10.1172/JCI75876
Survivin-specific T cell receptor targets tumor but not T cells
Abstract
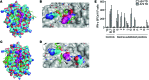
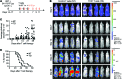
Survivin is a tumor-associated antigen (TAA) that inhibits apoptosis and is widely overexpressed in cancer cells; therefore, survivin has potential as a target for cancer immunotherapy. Application of HLA-A2-restricted survivin-specific T cell receptors (TCRs) isolated from allogeneic HLA-mismatched TCR repertoires has, however, been impeded by the inability of these TCRs to distinguish healthy cells expressing low levels of survivin from cancer cells with high survivin expression levels. Here, we identified an HLA-A2-restricted survivin-specific TCR isolated from autologous TCR repertoires that targets tumor cells in vitro and in vivo but does not cause fratricidal toxicity. Molecular modeling of the TCR-peptide-HLA ternary complexes and alanine scanning revealed that the autologously derived TCRs had tighter interactions with the survivin peptide than did fratricidal TCRs. Similar recognition patterns were observed among 7 additional TAA-specific TCRs isolated from allogeneic versus autologous repertoires. Together, the results from this study indicate that maximal peptide recognition is key for TCR selectivity and likely critical for reducing unwanted off-target toxicities. Moreover, isolating TCRs from autologous repertoires to maximize TCR selectivity has potential as a useful strategy to identify and select other shared tumor- and self-antigen-specific TCRs and ensure selective antitumor activity.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM097207/GM/NIGMS NIH HHS/United States
- R01 CA142636/CA/NCI NIH HHS/United States
- T32 HL092332/HL/NHLBI NIH HHS/United States
- P01CA94237/CA/NCI NIH HHS/United States
- P30CA125123/CA/NCI NIH HHS/United States
- R01CA142636/CA/NCI NIH HHS/United States
- P50 CA126752/CA/NCI NIH HHS/United States
- P50CA126752/CA/NCI NIH HHS/United States
- P30 CA125123/CA/NCI NIH HHS/United States
- R01HL114564/HL/NHLBI NIH HHS/United States
- R01CA131027/CA/NCI NIH HHS/United States
- R01 CA131027/CA/NCI NIH HHS/United States
- P01 CA094237/CA/NCI NIH HHS/United States
- R01 HL114564/HL/NHLBI NIH HHS/United States
- T32 DK060445/DK/NIDDK NIH HHS/United States
- R01GM097207/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

