Remodelling the extracellular matrix in development and disease
- PMID: 25415508
- PMCID: PMC4316204
- DOI: 10.1038/nrm3904
Remodelling the extracellular matrix in development and disease
Abstract
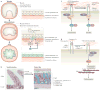
The extracellular matrix (ECM) is a highly dynamic structure that is present in all tissues and continuously undergoes controlled remodelling. This process involves quantitative and qualitative changes in the ECM, mediated by specific enzymes that are responsible for ECM degradation, such as metalloproteinases. The ECM interacts with cells to regulate diverse functions, including proliferation, migration and differentiation. ECM remodelling is crucial for regulating the morphogenesis of the intestine and lungs, as well as of the mammary and submandibular glands. Dysregulation of ECM composition, structure, stiffness and abundance contributes to several pathological conditions, such as fibrosis and invasive cancer. A better understanding of how the ECM regulates organ structure and function and of how ECM remodelling affects disease progression will contribute to the development of new therapeutics.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Bateman JF, Boot-Handford RP, Lamande SR. Genetic diseases of connective tissues: cellular and extracellular effects of ECM mutations. Nature Rev Genet. 2009;10:173–183. - PubMed
-
- Hynes RO, Naba A. Overview of the matrisome—an inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol. 2012;4:a004903. This review gives a complete list of ECM proteins that are part of the matrisome, and describes the ECM structure and function modifiers and the evolution of the matrisome. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

