Lipid-lowering efficacy of rosuvastatin
- PMID: 25415541
- PMCID: PMC6463960
- DOI: 10.1002/14651858.CD010254.pub2
Lipid-lowering efficacy of rosuvastatin
Abstract
Background: Rosuvastatin is one of the most potent statins and is currently widely prescribed. It is therefore important to know the dose-related magnitude of effect of rosuvastatin on blood lipids.
Objectives: Primary objective To quantify the effects of various doses of rosuvastatin on serum total cholesterol, low-density lipoprotein (LDL)-cholesterol, high-density lipoprotein (HDL)-cholesterol, non-HDL-cholesterol and triglycerides in participants with and without evidence of cardiovascular disease. Secondary objectives To quantify the variability of the effect of various doses of rosuvastatin.To quantify withdrawals due to adverse effects (WDAEs) in the randomized placebo-controlled trials.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL) Issue 10 of 12, 2014 in The Cochrane Library, MEDLINE (1946 to October week 5 2014), EMBASE (1980 to 2014 week 44), Web of Science Core Collection (1970 to 5 November 2014) and BIOSIS Citation Index (1969 to 31 October 2014). No language restrictions were applied.
Selection criteria: Randomized controlled and uncontrolled before-and-after trials evaluating the dose response of different fixed doses of rosuvastatin on blood lipids over a duration of three to 12 weeks.
Data collection and analysis: Two review authors independently assessed eligibility criteria for studies to be included and extracted data. WDAEs information was collected from the placebo-controlled trials.
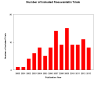
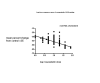
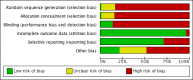
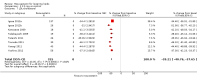
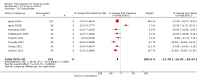
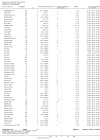
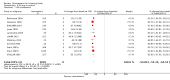
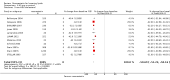
Main results: One-hundred and eight trials (18 placebo-controlled and 90 before-and-after) evaluated the dose-related efficacy of rosuvastatin in 19,596 participants. Rosuvastatin 10 to 40 mg/day caused LDL-cholesterol decreases of 46% to 55%, when all the trials were combined using the generic inverse variance method. The quality of evidence for these effects is high. Log dose-response data over doses of 1 to 80 mg, revealed strong linear dose-related effects on blood total cholesterol, LDL-cholesterol and non-HDL-cholesterol. When compared to atorvastatin, rosuvastatin was about three-fold more potent at reducing LDL-cholesterol. There was no dose-related effect of rosuvastatin on blood HDL-cholesterol, but overall, rosuvastatin increased HDL by 7%. There is a high risk of bias for the trials in this review, which would affect WDAEs, but unlikely to affect the lipid measurements. WDAEs were not statistically different between rosuvastatin and placebo in 10 of 18 of these short-term trials (risk ratio 0.84; 95% confidence interval 0.48 to 1.47).
Authors' conclusions: The total blood total cholesterol, LDL-cholesterol and non-HDL-cholesterol-lowering effect of rosuvastatin was linearly dependent on dose. Rosuvastatin log dose-response data were linear over the commonly prescribed dose range. Based on an informal comparison with atorvastatin, this represents a three-fold greater potency. This review did not provide a good estimate of the incidence of harms associated with rosuvastatin because of the short duration of the trials and the lack of reporting of adverse effects in 44% of the placebo-controlled trials.
Conflict of interest statement
None known.
Figures



































































Update of
References
References to studies included in this review
Agouridis 2011 {published data only}
-
- Agouridis AP, Tsimihodimos V, Filippatos TD, Dimitriou AA, Tellis Costantinos C, Elisaf MS, et al. The effects of rosuvastatin alone or in combination with fenofibrate or omega 3 fatty acids on inflammation and oxidative stress in patients with mixed dyslipidemia. Expert Opinion on Pharmacotherapy 2011;12(17):2605‐11. [MEDLINE: ] - PubMed
-
- Agouridis AP, Tsimihodimos V, Filippatos TD, Tselepis AD, Elisaf MS. High doses of rosuvastatin are superior to low doses of rosuvastatin plus fenofibrate or n‐3 fatty acids in mixed dyslipidemia. Lipids 2011;46(6):521‐8. [MEDLINE: ] - PubMed
Andreou 2010 {published data only}
-
- Andreou I, Tousoulis D, Miliou A, Tentolouris C, Zisimos K, Gounari P, et al. Effects of rosuvastatin on myeloperoxidase levels in patients with chronic heart failure: A randomized placebo‐controlled study. Atherosclerosis 2010;210(1):194‐8. [MEDLINE: ] - PubMed
-
- Tousoulis D, Andreou I, Tentolouris C, Antoniades C, Papageorgiou N, Gounari P, et al. Comparative effects of rosuvastatin and allopurinol on circulating levels of matrix metalloproteinases and tissue inhibitors of metalloproteinases in patients with chronic heart failure. International Journal of Cardiology 2010;145(3):438‐43. [MEDLINE: ] - PubMed
-
- Tousoulis D, Andreou I, Tsiatas M, Miliou A, Tentolouris C, Siasos G, et al. Effects of rosuvastatin and allopurinol on circulating endothelial progenitor cells in patients with congestive heart failure: The impact of inflammatory process and oxidative stress. Atherosclerosis 2011;214(1):151‐7. [MEDLINE: ] - PubMed
ANDROMEDA 2007 {published data only}
-
- Betteridge DJ, Gibson J, Sager PT. Comparison of effectiveness of Rosuvastatin versus Atorvastatin on the achievement of combined c‐reactive protein (< 2 mg/L) and low‐density lipoprotein cholesterol (< 70 mg/dl) targets in patients with type 2 diabetes Mellitus (from the ANDROMEDA study). American Journal of Cardiology 2007;100(8):1245‐8. [MEDLINE: ] - PubMed
-
- Betteridge DJ, Gibson JM. Effects of rosuvastatin on lipids, lipoproteins and apolipoproteins in the dyslipidaemia of diabetes. Diabetic Medicine 2007;24(5):541‐9. [MEDLINE: ] - PubMed
ARIES 2006 {published data only}
-
- AstraZeneca. A 6‐week, randomized, open‐label, comparative study to evaluate the efficacy and safety of rosuvastatin versus atorvastatin in the treatment of hypercholesterolaemia in African American subjects. (ARIES). http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐... (accessed 07 July 2004). [Study code D3560L00022/4522US0002]
-
- Ferdinand KC, Clark LT, Watson KE, Neal RC, Brown CD, Kong BW, et al. Comparison of efficacy and safety of rosuvastatin versus atorvastatin in African‐American patients in a six‐week trial. American Journal of Cardiology 2006;97(2):229‐35. [MEDLINE: ] - PubMed
-
- Fonseca FA, Izar MCO, Silva MA, Karapanos A, Schuster H, Singh D. Rosuvastatin may be more effective than atorvastatin in African‐Americans with hypercholesterolemia. Evidence‐based Cardiovascular Medicine 2006;10(2):96‐100. [MEDLINE: ] - PubMed
AstraZeneca 2010a {published data only}
-
- AstraZeneca. A randomised, double‐blind trial to compare the efficacy of rosuvastatin 5 and 10 mg to atorvastatin 10 mg in the treatment of high risk patients with hypercholesterolemia followed by an open label treatment period with rosuvastatin up‐titrated to the maximum dose of 20 mg for those patients who do not achieve goal. http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐... (accessed 02 June 2010). [Study code D356FC00007]
-
- ClinicalTrials.gov. Compare the efficacy of rosuvastatin to atorvastatin in high risk patients with yypercholesterolemia. http://www.clinicaltrials.gov/ct2/show/results/NCT00683618?term‐D356FC00... (accessed 09 July 2010). [Study code NCT00683618]
AstraZeneca 2010b {published data only}
-
- AstraZeneca. A double blind, double dummy, phase IV, randomized, multicenter, parallel group, placebo control trial to evaluate the effect of rosuvastatin on triglycerides levels in Mexican hypertriglyceridemic patients. http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐... (accessed 28 March 2010). [Study code DM‐CRESTOR‐0002/D3560L00075]
-
- ClinicalTrials.gov. Effect of rosuvastatin on triglyceride levels in Mexican hypertriglyceridemic patients. http://clinicaltrials.gov/ct2/show/results/NCT00473655?sect=X43da987601 (accessed 10 May 2010). [ Study code NCT00473655]
ASTRO‐2 2009 {published data only}
ASTRONOMER 2010 {published data only}
-
- Capoulade R, Clavel MA, Dumesnil JG, Chan KL, Teo KK, Tam JW, et al. Impact of metabolic syndrome on progression of aortic stenosis: influence of age and statin therapy. Journal of the American College of Cardiology 2012;60(3):216‐23. [MEDLINE: ] - PubMed
-
- Capoulade R, Clavel MA, Dumesnil JG, Chan KL, Teo KK, Tam JW, et al. Insulin resistance and LVH progression in patients with calcific aortic stenosis: a substudy of the ASTRONOMER trial. JACC. Cardiovascular Imaging 2013;6(2):165‐74. [MEDLINE: ] - PubMed
-
- Chan K‐L, Teo K, Tam J, Dumesnil JG. Rationale, design, and baseline characteristics of a randomized trial to assess the effect of cholesterol lowering on the progression of aortic stenosis. The Aortic Stenosis Progression Observation: Measuring Effects of Rosuvastatin (ASTRONOMER) trial. American Heart Journal 2007;153(6):925‐31. [MEDLINE: ] - PubMed
-
- Chan KL, Dumesnil JG, Tam J, Ni A, Teo K. Effect of rosuvastatin on C‐reactive protein and progression of aortic stenosis. American Heart Journal 2011;161(6):1133‐9. [MEDLINE: ] - PubMed
-
- Chan KL, Teo K, Dumesnil JG, Ni A, ASTRONOMER Study Group. Effect of lipid lowering with rosuvastatin on progression of aortic stenosis results of the Aortic Stenosis Progression Observation: Measuring Effects of Rosuvastatin (ASTRONOMER) Trial. Circulation 2010;121(2):306‐14. [MEDLINE: ] - PubMed
ATOROS 2006 {published data only}
-
- Milionis HJ, Rizos E, Kostapanos M, Filippatos TD, Elisaf MS. Treating to target patients with primary hyperlipidaemia: comparison of the effects of ATOrvastatin and ROSuvastatin (the ATOROS study). Current Medical Research & Opinion 2006;22(6):1123‐31. [MEDLINE: ] - PubMed
Ballantyne 2003 {published data only}
-
- Ballantyne CM, Stein EA, Paoletti R, Southworth H, Blasetto JW, Barter P, et al. Efficacy of rosuvastatin 10 mg in patients with the metabolic syndrome. American Journal of Cardiology 2003;91 Suppl 1(5):25C‐28C. [MEDLINE: ] - PubMed
Ballantyne 2004 {published data only}
-
- Ballantyne CM, Miller E, Chitra R. Efficacy and safety of rosuvastatin alone and in combination with cholestyramine in patients with severe hypercholesterolemia: A randomized, open‐label, multicenter trial. Clinical Therapeutics 2004;26(11):1855‐64. [MEDLINE: ] - PubMed
Bellia 2010 {published data only}
-
- Bellia A, Rizza S, Galli A, Fabiano R, Donadel G, Lombardo MF, et al. Early vascular and metabolic effects of rosuvastatin compared with simvastatin in patients with type 2 diabetes. Atherosclerosis 2010;210(1):199‐201. [MEDLINE: ] - PubMed
Briseno 2010 {published data only}
-
- Briseno GG, Mino‐Leon D. Cost‐effectiveness of rosuvastatin versus ezetimibe/simvastatin in managing dyslipidemic patients in Mexico. Current Medical Research and Opinion 2010;26(5):1075‐81. [MEDLINE: ] - PubMed
Brown 2002 {published data only}
-
- AstraZeneca. A randomized, double‐blind, multicenter trial to compare the short‐term and long‐term efficacy and safety of ZD4522 5 and 10 mg, simvastatin 20 mg, and pravastatin 20 mg in the treatment of subjects with hypercholesterolemia. http://astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐tria... (accessed 30 March 2001). [ Study code 4522IL/0028]
-
- Benner JS, Smith TW, Klingman D, Tierce JC, Mullins CD, Pethick N, et al. Cost‐effectiveness of rosuvastatin compared with other statins from a managed care perspective. Value in Health : the Journal of the International Society for Pharmacoeconomics and Outcomes Research. United States: ValueMedics Research, LLC, Arlington, VA, and University of Maryland, Baltimore, MD, USA. josh.benner@valuemedics.com, 2005; Vol. 8, issue 6:618‐28. [MEDLINE: ] - PubMed
-
- Blasetto JW, Stein EA, Brown WV, Chitra R, Raza A. Efficacy of rosuvastatin compared with other statins at selected starting doses in hypercholesterolemic patients and in special population groups. American Journal of Cardiology. United States: AstraZeneca LP, Wilmington, Delaware 19850, USA. james.blasetto@astrazeneca.com, 2003; Vol. 91, issue 5A:3C‐10C. [MEDLINE: ] - PubMed
-
- Brown VW. A 52‐week trial of rosuvastatin versus pravastatin and simvastatin in patients with primary hypercholesterolemia. International Journal of Clinical Practice 2002;Suppl 124:12. [CENTRAL: CN‐00858312 NEW]
-
- Brown WV, Bays HE, Hassman DR, McKenney J, Chitra R, Hutchinson H, et al. Efficacy and safety of rosuvastatin compared with pravastatin and simvastatin in patients with hypercholesterolemia: A randomized, double‐blind, 52‐week trial. American Heart Journal 2002;144(6):1036‐43. [MEDLINE: ] - PubMed
CAP‐Chol 2009 {published data only}
-
- AstraZeneca. Evaluation of the efficacy and safety of rosuvastatin 5 mg versus pravastatin 40 mg and atorvastatin 10 mg in type IIa and IIb hypercholesterolaemic patients. http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐... (accessed 16 September 2009). [Study code D3560L00068/NCT00631189]
Capuzzi 2003 {published data only}
-
- Capuzzi DM, Morgan JM, Weiss RJ, Chitra RR, Hutchinson HG, Cressman MD. Beneficial effects of rosuvastatin alone and in combination with extended‐release niacin in patients with a combined hyperlipidemia and low high‐density lipoprotein cholesterol levels. American Journal of Cardiology 2003;91(11):1304‐10. [MEDLINE: ] - PubMed
Catapano 2006 {published data only}
-
- Catapano AL, Davidson MH, Ballantyne CM, Brady WE, Gazzara RA, Tomassini JE, et al. Lipid‐altering efficacy of the ezetimibe/simvastatin single tablet versus rosuvastatin in hypercholesterolemic patients. Current Medical Research and Opinion 2006;22(10):2041‐53. [MEDLINE: ] - PubMed
-
- ClinicalTrials.gov. A multicenter, randomized, double‐blind study to evaluate the lipid‐altering efficacy and safety of the ezetimibe/simvastatin combination tablet versus rosuvastatin in patients with primary hypercholesterolemia. http://clinicaltrials.gov/show/NCT00090298 (accessed 24 May 2013). [Study code NCT00090298]
-
- Davidson MH, Abate N, Ballantyne CM, Catapano AL, Xu X, Lin J, et al. Ezetimibe/simvastatin compared with atorvastatin or rosuvastatin in lowering to specified levels both LDL‐C and each of five other emerging risk factors for coronary heart disease: Non‐HDL‐cholesterol, TC/HDL‐C, apolipoprotein B, apo‐B/apo‐A‐I, or C‐reactive protein. Journal of Clinical Lipidology 2008;2(6):436‐46. [EMBASE: 2008584795] - PubMed
Celik 2012 {published data only}
-
- Celik O, Acbay O. Effects of metformin plus rosuvastatin on hyperandrogenism in polycystic ovary syndrome patients with hyperlipidemia and impaired glucose tolerance. Journal of Endocrinological Investigation 2012;35(10):905‐10. [MEDLINE: ] - PubMed
Chiang 2008 {published data only}
-
- Chiang CE, Huang SS, Sung SH. Efficacy and safety of rosuvastatin in Taiwanese patients. Journal of the Chinese Medical Association: JCMA 2008;71(3):113‐8. [EMBASE: 2008210915] - PubMed
Coban 2008 {published data only}
-
- Coban E, Afacan B. The effect of rosuvastatin treatment on the mean platelet volume in patients with uncontrolled primary dyslipidemia with hypolipidemic diet treatment. Platelets 2008;19(2):111‐4. [MEDLINE: ] - PubMed
Coen 2009 {published data only}
-
- ClinicalTrials.gov. Phase 4 clinical trial to examine the role of rosuvastatin and exercise treatment in modulating inflammatory response in hypercholesterolemic subjects. http://clinicaltrials.gov/show/NCT00295373 (accessed 19 April 2007). [Study code NCT00295373]
-
- Coen PM. Statin treatment and exercise: Is there an additive anti‐inflammatory effect?. ProQuest Dissertations and Theses 2008. [DOI: ]
-
- Coen PM, Flynn MG, Markofski MM, Pence BD, Hannemann RE. Adding exercise to rosuvastatin treatment: influence on C‐reactive protein, monocyte toll‐like receptor 4 expression, and inflammatory monocyte (CD14+CD16+) population. Metabolism: Clinical and Experimental 2010; Vol. 59, issue 12:1775‐83. [MEDLINE: ] - PubMed
-
- Coen PM, Flynn MG, Markofski MM, Pence BD, Hannemann RE. Adding exercise training to rosuvastatin treatment: influence on serum lipids and biomarkers of muscle and liver damage. Metabolism: Clinical and Experimental 2009;58(7):1030‐8. [MEDLINE: ] - PubMed
COMETS 2005 {published data only}
-
- ClinicalTrials.gov. A 12 week randomized, double‐blind, force‐titration, parallel group, multi centre, phase IIIb study to compare the efficacy of rosuvastatin with atorvastatin and placebo in the treatment of non‐diabetic, non‐atheroscleric, metabolic syndrome subjects with raised LDL‐C and a 10 year risk of CHD >10%. http://clinicaltrials.gov/show/NCT00654485 (accessed 13 March 2009). [Study code 4522IL/0069/ D3560C00069/ NCT00654485]
-
- Stalenhoef AF, Ballantyne CM, Sarti C, Murin J, Tonstad S, Rose H, et al. A comparative study with rosuvastatin in subjects with metabolic syndrome: results of the COMETS study. European Heart Journal 2005;26(24):2664‐72. [MEDLINE: ] - PubMed
CORALL 2005 {published data only}
-
- Franken A. Cholesterol‐lowering effects of rosuvastatin compared with atorvastatin in patients with type 2 diabetes. Atherosclerosis Supplements 2004;5(1):118. [MEDLINE: ]
-
- Simsek S, Schalkwijk CG, Wolffenbuttel BH, Simsek S, Schalkwijk CG, Wolffenbuttel BHR. Effects of rosuvastatin and atorvastatin on glycaemic control in Type 2 diabetes‐‐‐the CORALL study. Diabetic Medicine 2012;29(5):628‐31. [MEDLINE: ] - PubMed
-
- Wolffenbuttel BH, Franken AA, Vincent HH, Dutch Corall Study Group. Cholesterol‐lowering effects of rosuvastatin compared with atorvastatin in patients with type 2 diabetes ‐‐ CORALL study. Journal of Internal Medicine 2005;257(6):531‐9. [MEDLINE: ] - PubMed
Davidson 2002 {published data only}
-
- AstraZeneca. A 12‐week, randomized, double‐blind, placebo‐controlled, multicenter trial to evaluate the efficacy and safety of ZD4522 5mg and 10 mg and atorvastatin 10 mg in the treatment of subjects with hypercholesterolemia. http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐... (access 24 August 2000). [Study code 4522IL/0024]
-
- Blasetto JW, Stein EA, Brown WV, Chitra R, Raza A. Efficacy of rosuvastatin compared with other statins at selected starting doses in hypercholesterolemic patients and in special population groups. American Journal of Cardiology 2003;91(5A):3C‐10C. [MEDLINE: ] - PubMed
-
- Davidson M. Rosuvastatin is more effective than atorvastatin at improving the lipid profiles of patients with primary hypercholesterolaemia. Atherosclerosis Supplements 2001;2(2):38. [MEDLINE: ]
-
- Davidson M, Ma P, Stein EA, Gotto AM Jr, Raza A, Chitra R, Hutchinson H. Comparison of effects on low‐density lipoprotein cholesterol and high‐density lipoprotein cholesterol with rosuvastatin versus atorvastatin in patients with type IIa or IIb hypercholesterolemia. American Journal of Cardiology 2002;89(3):268‐75. [MEDLINE: ] - PubMed
-
- Prescott LM. Highlights of the American College of Cardiology's 50th Annual Scientific Session. P and T 2001;26(6):330‐2. [EMBASE: 2001216890]
DISCOVERY‐Asia 2007 {published data only}
-
- AstraZeneca. An open‐label, randomized, multi‐centre, phase IIIb/IV, parallel group study to compare the efficacy and safety of rosuvastatin and atorvastatin in subjects with type IIa and IIb hypercholesterolemia (DISCOVERY). Clinical Study Report Synopsis (access 10 February 2007). [Code study D3560L00009/NCT00241488]
-
- Zhu JR, Tomlinson B. A randomised study comparing the efficacy and safety of rosuvastatin with atorvastatin for achieving lipid goals in clinical practice in Asian patients at high risk of cardiovascular disease (DISCOVERY‐Asia study). Current Medical Research and Opinion 2007;23(12):3055‐68. [MEDLINE: ] - PubMed
Dulay 2009 {published data only}
Durrington 2004 {published data only}
-
- AstraZeneca. A 24‐week, randomised, multicentre trial to evaluate the efficacy and safety of ZD4522 and fenofibrate, alone and in various combinations, in the treatment of type IIb and IV hyperlipidaemia associated with type 2 diabetes mellitus. http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐... (accessed 06 March 2001). [Study code 4522IL/0036]
-
- Durrington PN, Tuomilehto J, Hamann A, Kallend D, Smith K. Rosuvastatin and fenofibrate alone and in combination in type 2 diabetes patients with combined hyperlipidaemia. Diabetes Research and Clinical Practice 2004;64(2):137‐51. [MEDLINE: ] - PubMed
Dzhaiani 2008 {published data only}
-
- Dzhaiani NA, Ilyina EV, Kochetov AG, Tereshchenko SN. Lipid‐lowering and pleiotropic effects of rosuvastatin in patients with acute myocardial infarction. Cardiovascular Therapy and Prevention 2008;7(7):91‐7. [CENTRAL: CN‐00865232 NEW]
ECLIPSE 2008 {published data only}
-
- AstraZeneca. A 24‐week, randomised, open‐label, parallel‐group, multicentre study which compares the efficacy and safety of rosuvastatin 10, 20 and 40 mg with atorvastatin 10, 20, 40 and 80 mg when force‐titrated in the treatment of patients with primary hypercholesterolemia and either a history ofcoronary heart disease (CHD) or clinical evidence of atherosclerosis or a CHD risk equivalent (10‐year risk score >20%) ECLIPSE ‐ An Evaluation to Compare Lipid‐lowering effects of rosuvastatin and atorvastatin In force‐titrated patients: a Prospective Study of Efficacy and tolerability. http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐... (accessed 03 February 2006). [Study code D3569C00002]
-
- Faergeman O. Efficacy and tolerability of rosuvastatin and atorvastatin when force‐titrated in patients with primary hypercholesterolemia: results from the ECLIPSE study. Cardiology 2008;111(4):219‐28. [MEDLINE: ] - PubMed
EFFORT 2011 {published data only}
-
- AstraZeneca. Open‐labelled, single arm, phase IV clinical study to evaluate the impact of rosuvastatin on lipid levels in patients with metabolic syndrome. http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐... (access 07 March 2011). [Study code D3560L00079]
-
- ClincialTrials.gov. Effect of Crestor (rosuvastatin) on lipid levels in patients with metabolic syndrome (EFFORT). http://clinicaltrials.gov/show/NCT00815659 (access 29 August 2011). [Study code NCT00815659]
Erbs 2011 {published data only}
-
- Erbs S, Beck EB, Linke A, Adams V, Gielen S, Krankel N, et al. High‐dose rosuvastatin in chronic heart failure promotes vasculogenesis, corrects endothelial function, and improves cardiac remodeling ‐ Results from a randomized, double‐blind, and placebo‐controlled study. International Journal of Cardiology 2011;146(1):56‐63. [MEDLINE: ] - PubMed
EXPLORER 2007 {published data only}
-
- AstraZeneca. A 6 wk open‐label, randomised, multicentre, phase iiib, parallel group study to compare the safety & efficacy of rosuvastatin 40 mg in comb.with ezetimibe 10mg in subjects with hypercholesterolaemia & CHD or atherosclerosis or a CHD risk equiv. (10 yr risk score >20%). http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐... (accessed 06 January 2006. [Study code D3569C00006/NCT00653445]
-
- Ballantyne CM, Weiss R, Moccetti T, Vogt A, Eber B, Sosef F, Duffield E. Efficacy and safety of rosuvastatin 40 mg alone or in combination with ezetimibe in patients at high risk of cardiovascular disease (results from the EXPLORER study). American Journal of Cardiology 2007;99(5):673‐80. [MEDLINE: ] - PubMed
-
- Lepor NE. Key findings from the 2006 World Congress of Cardiology: A summary from the Joint Meeting of the European Society of Cardiology and the World Heart Federation, September 2‐5, 2006, Barcelona, Spain. Reviews in Cardiovascular Medicine 2007;8(2):90‐100. [EMBASE: 2007331650]
Florentin 2013 {published data only}
-
- Florentin M, Liberopoulos EN, Rizos CV, Kei AA, Liamis G, Kostapanos MS, et al. Colesevelam plus rosuvastatin 5mg/day versus rosuvastatin 10mg/day alone on markers of insulin resistance in patients with hypercholesterolemia and impaired fasting glucose. Metabolic Syndrome & Related Disorders 2013;11(3):152‐6. - PubMed
Gao 2007 {published data only}
-
- Gao RL. The efficacy and safety of rosuvastatin on treating patients with hypercholesterolemia in Chinese: a randomized, double‐blind, multi‐center clinical trial. Zhonghua xin xue guan bing za zhi [Chinese Journal of Cardiovascular Diseases] 2007;35(3):207‐11. [EMBASE: 17582281] - PubMed
Gomez‐Garcia 2007 {published data only}
-
- Gomez‐Garcia A, Martinez Torres G, Ortega‐Pierres LE, Rodriguez‐Ayala E, Alvarez‐Aguilar C. Rosuvastatin and metformin decrease inflammation and oxidative stress in patients with hypertension and dyslipidemia. Revista Espanola de Cardiologia 2007;60(12):1242‐9. [MEDLINE: ] - PubMed
GRAVITY 2009 {published data only}
-
- ClinicalTrials.gov. 12‐week open‐label, phase IIIb comparing efficacy and safety of rosuvastatin (CRESTOR) in combination with ezetimibe (GRAVITY). http://clinicaltrials.gov/ct2/show/results/NCT00525824 (accessed 03 September 2009). [Study code NCT0052584/D356FC00003]
Guo 2012 {published data only}
-
- Guo Chunhong. Efficacy and safety of rosuvastatin in treatment of patients with ischemic stroke for secondary prevention of stroke. Chinese Journal of Hospital Pharmacy [Zhongguo yiyuan yaoxue zazhi] 2012;32(3):211‐3. [CENTRAL: CN‐00858141 NEW]
Han 2008 {published data only}
-
- Han Hui, Xue Jing, Zhang Jing, Xu Fang, Wang Yu. Efficacy and safety of rosuvastatin and atorvastatin in aged patients with hypercholesterolemia. Zhongguo Xinyao yu Linchuang Zazhi 2008;27(2):120‐3. [CENTRAL: CN‐00865233 NEW]
HeFH 2003 {published data only}
-
- AstraZeneca. A 24‐week, randomised, double‐blind, multicentre, multinational trial to evaluate the efficacy and safety of ZD4522 and atorvastatin in the treatment of subjects with heterozygous familial hypercholesterolaemia. http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐... (accessed 09 February 2001. [Study code 4522IL/0030]
-
- Stein EA, Strutt K, Southworth H, Diggle PJ, Miller E. Comparison of rosuvastatin versus atorvastatin in patients with heterozygous familial hypercholesterolemia. American Journal of Cardiology 2003;92(11):1287‐93. [MEDLINE: ] - PubMed
Her 2010 {published data only}
-
- Her AY, Kim JY, Kang SM, Choi D, Jang Y, Chung N, et al. Effects of atorvastatin 20 mg, rosuvastatin 10 mg, and atorvastatin/ ezetimibe 5 mg/5 mg on lipoproteins and glucose metabolism. Journal of Cardiovascular Pharmacology and Therapeutics 2010;15(2):167‐74. [MEDLINE: ] - PubMed
Hunninghake 2004 {published data only}
-
- AstraZeneca. A 12‐week, multicenter, randomized, double‐blind, placebo‐controlled trial to evaluate the efficacy and safety of ZD4522 5 to 80 mg in the treatment of subjects with hypertriglyceridemia. http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐... (accessed 15 January 2001). [Study code 4522IL/0035]
-
- Hunninghake D, Chitra R, Simonson S, Schneck D. Rosuvastatin markedly improved the atherogenic lipid profile in hypertriglyceridaemic patients. European Heart Journal 2001;22 Abstract Supplement:270. [BIOSIS:PREV200200091177]
-
- Hunninghake DB. Effects of rosuvastatin on serum lipids and lipid subfractions in patients with hypertriglyceridemia. International Journal of Clinical Practice 2002;Suppl 124:10. [CENTRAL: CN‐00866593 NEW]
-
- Hunninghake DB. Treatment of hypertriglyceridemic patients with rosuvastatin. Diabetes 2001;50 Suppl 2:A143. [CENTRAL: CN‐00866594 NEW]
-
- Hunninghake DB, Stein EA, Bays HE, Rader DJ, Chitra RR, Simonson SG, et al. Rosuvastatin improves the atherogenic and atheroprotective lipid profiles in patients with hypertriglyceridemia. Coronary Artery Disease 2004;15(2):115‐23. [MEDLINE: ] - PubMed
Igase 2012a {published data only}
-
- Igase M, Kohara K, Katagi R, Yamashita S, Fujisawa M, Miki T. Predictive value of the low‐density lipoprotein cholesterol to high‐density lipoprotein cholesterol ratio for the prevention of stroke recurrence in Japanese patients treated with rosuvastatin [I]. Clinical Drug Investigation 2012;32(8):513‐21. [MEDLINE: ] - PubMed
Igase 2012b {published data only}
-
- Igase M, Kohara K, Tabara Y, Nagai T, Ochi N, Kido T, et al. Low‐dose rosuvastatin improves the functional and morphological markers of atherosclerosis in asymptomatic postmenopausal women with dyslipidemia. Menopause 2012;19(12):1294‐9. [MEDLINE: ] - PubMed
IRIS 2007 {published data only}
-
- ClinicalTrials.gov. A 6‐week, randomized,open‐label, comparative study to evaluate the efficacy and safety of rosuvastatin and atorvastatin in the treatment of hypercholesterolaemia in South Asian subjects. http://clinicaltrials.gov/show/NCT00654225 (accessed 13 March 2009). [study code NCT00654225]
-
- Deedwania PC, Gupta M, Stein M, Ycas J, Gold A. Comparison of rosuvastatin versus atorvastatin in South‐Asian patients at risk of coronary heart disease (from the IRIS Trial). American Journal of Cardiology 2007;99(11):1538‐43. [MEDLINE: ] - PubMed
JART 2012 {published data only}
-
- Nohara R, Daida H, Hata M, Kaku K, Kawamori R, Kishimoto J, et al. Effect of intensive lipid‐lowering therapy with rosuvastatin on progression of carotid intima‐media thickness in Japanese patients. Circulation Journal 2012;76(1):221‐9. [MEDLINE: ] - PubMed
-
- Yamazaki T, Nohara R, Daida H, Hata M, Kaku K, Kawamori R, et al. Intensive lipid‐lowering therapy for slowing progression as well as inducing regression of atherosclerosis in Japanese patients: subanalysis of the JART study. International Heart Journal 2013;54(1):33‐9. [MEDLINE: ] - PubMed
Jing 2013 {published data only}
-
- Jing S, Sun N ‐L, Li X ‐Y, Hua Q, Wang L, Li Z ‐Q, et al. Efficacy and safety of rosuvastatin in the treatment of hypercholesterolemia. Chinese Journal of New Drugs 2013;22(8):937‐40.
Jones 2009 {published data only}
-
- Bays HE, Jones PH, Mohiuddin SM, Kelly MT, Sun H, Setze CM, et al. Long‐term safety and efficacy of fenofibric acid in combination with statin therapy for the treatment of patients with mixed dyslipidemia. Journal of Clinical Lipidology 2008;2(6):426‐35. [EMBASE: 2008584794] - PubMed
-
- ClinicalTrials.gov. Evaluate safety and efficacy of ABT‐335 in combination with rosuvastatin calcium in subjects with multiple abnormal lipid levels in the blood. http://clinicaltrials.gov/show/NCT00300482 (accessed 03 June 2009). [Study code NCT00300482]
-
- Jones PH, Bays HE, Davidson MH, Kelly MT, Buttler SM, Setze CM, et al. Evaluation of a new formulation of fenofibric acid, ABT‐335, co‐administered with statins : study design and rationale of a phase III clinical programme. Clinical Drug Investigation 2008;28(10):625‐34. [MEDLINE: ] - PubMed
-
- Jones PH, Cusi K, Davidson MH, Kelly MT, Setze CM, Thakker K, et al. Efficacy and safety of fenofibric acid co‐administered with low‐ or moderate‐dose statin in patients with mixed dyslipidemia and type 2 diabetes mellitus: results of a pooled subgroup analysis from three randomized, controlled, double‐blind trials. American Journal of Cardiovascular Drugs : drugs, devices, and other interventions 2010;10(2):73‐84. [MEDLINE: ] - PubMed
Kanazawa 2009 {published data only}
-
- Kanazawa I, Yamaguchi T, Yamauchi M, Sugimoto T. Rosuvastatin increased serum osteocalcin levels independent of its serum cholesterol‐lowering effect in patients with type 2 diabetes and hypercholesterolemia. Internal Medicine 2009;48(21):1869‐73. [MEDLINE: ] - PubMed
Kim 2013 {published data only}
Koh 2013 {published data only}
-
- Koh KK, Quon MJ, Sakuma I, Han SH, Choi H, Lee K, et al. Differential metabolic effects of rosuvastatin and pravastatin in hypercholesterolemic patients. International Journal of Cardiology 2013;166(2):509‐15. - PubMed
Kostapanos 2006 {published data only}
-
- Kostapanos MS, Milionis HJ, Gazi I, Kostara C Bairaktari ET, Elisaf M. Rosuvastatin increases alpha‐1 microglobulin urinary excretion in patients with primary dyslipidemia. Journal of Clinical Pharmacology 2006;46(11):1337‐43. [MEDLINE: ] - PubMed
Kostapanos 2007a {published data only}
-
- Kostapanos MS, Milionis HJ, Filippatos TD, Nakou ES, Bairaktari ET, Tselepis AD, et al. A 12‐week, prospective, open‐label analysis of the effect of rosuvastatin on triglyceride‐rich lipoprotein metabolism in patients with primary dyslipidemia. Clinical Therapeutics 2007;29(7):1403‐14. [MEDLINE: ] - PubMed
Kostapanos 2007b {published data only}
-
- Kostapanos MS, Milionis HJ, Saougos VG, Lagos KG, Kostara C, Bairaktari ET, et al. Dose‐dependent effect of rosuvastatin treatment on urinary protein excretion. Journal of Cardiovascular Pharmacology and Therapeutics 2007;12(4):292‐7. [MEDLINE: ] - PubMed
Kostapanos 2008a {published data only}
-
- Kostapanos MS, Derdemezis CS, Filippatos TD, Milionis HJ, Kiortsis DN, Tselepis AD, et al. Effect of rosuvastatin treatment on plasma visfatin levels in patients with primary hyperlipidemia. European Journal of Pharmacology 2008;578(2‐3):249‐52. [MEDLINE: ] - PubMed
Kostapanos 2008b {published data only}
-
- Kostapanos MS, Milionis HJ, Lagos KG, Rizos CB, Tselepis AD, Elisaf MS. Baseline triglyceride levels and insulin sensitivity are major determinants of the increase of LDL particle size and buoyancy induced by rosuvastatin treatment in patients with primary hyperlipidemia. European Journal of Pharmacology 2008;590(1‐3):327‐32. [MEDLINE: ] - PubMed
Kostapanos 2009 {published data only}
-
- Kostapanos MS, Milionis HJ, Filippatos TD, Christogiannis LG, Bairaktari ET Tselepis AD, et al. Dose‐dependent effect of rosuvastatin treatment on HDL‐subfraction phenotype in patients with primary hyperlipidemia. Journal of Cardiovascular Pharmacology and Therapeutics 2009;14(1):5‐13. [MEDLINE: ] - PubMed
Lamendola 2005 {published data only}
-
- Lamendola C, Abbasi F, Chu JW, Hutchinson H, Cain V, Leary E, et al. Comparative effects of rosuvastatin and gemfibrozil on glucose, insulin, and lipid metabolism in insulin‐resistant, nondiabetic patients with combined dyslipidemia. American Journal of Cardiology 2005;95(2):189‐93. [MEDLINE: ] - PubMed
Liberopoulos 2013 {published data only}
-
- Liberopoulos EN, Moutzouri E, Rizos CV, Barkas F, Liamis G, Elisaf MS, et al. Effects of manidipine plus rosuvastatin versus olmesartan plus rosuvastatin on markers of insulin resistance in patients with impaired fasting glucose, hypertension, and mixed dyslipidemia. Journal of Cardiovascular Pharmacology & Therapeutics 2013;18(2):113‐8. [MEDLINE: ] - PubMed
Lu 2004 {published data only}
-
- Lu T‐M, Ding Y‐A, Leu H‐B, Yin W‐H, Sheu WH‐H, Chu K‐M. Effect of rosuvastatin on plasma levels of asymmetric dimethylarginine in patients with hypercholesterolemia. American Journal of Cardiology 2004;94(2):157‐61. [MEDLINE: ] - PubMed
Lui 2007 {published data only}
-
- Lui SH. Pharmacogenetic and environmental determinants of response to HMG‐CoA reductase inhibitors. Hong Kong: ProQuest, UMI Dissertations, 2007.
LUNAR 2012 {published data only}
-
- AstraZeneca. A 12‐week randomized, open‐label 3‐arm, parallel group, multicenter Phase IIIb study comparing efficacy and safety of rosuvastatin 20mg and 40mg with that of atorvastatin 80 mg in subjects with acute coronary syndromes (LUNAR). http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐... (accessed 22 April 2008). [Study code D3560L00021/4522US/0001]
-
- Ballantyne CM, Pitt B, Loscalzo J, Cain VA, Raichlen JS. Alteration of relation of atherogenic lipoprotein cholesterol to apolipoprotein B by intensive statin therapy in patients with acute coronary syndrome (from the Limiting UNdertreatment of lipids in ACS With Rosuvastatin [LUNAR] Trial). American Journal of Cardiology 2013;111(4):506‐9. [MEDLINE: ] - PubMed
-
- Pitt B, Loscalzo J, Monyak J, Miller E, Raichlen J. Comparison of lipid‐modifying efficacy of rosuvastatin versus atorvastatin in patients with acute coronary syndrome (from the LUNAR study). American Journal of Cardiology 2012;109(9):1239‐46. [MEDLINE: ] - PubMed
-
- Pitt B, Loscalzo J, Ycas J, Raichlen JS. Lipid levels after acute coronary syndromes. Journal of the American College of Cardiology 2008;51(15):1440‐5. [EMBASE: 2008161797] - PubMed
Mabuchi 2004 {published data only}
-
- Mabuchi H, Nohara A, Higashikata T, Ueda K, Bujo H, Matsushima T, et al. Clinical efficacy and safety of rosuvastatin in Japanese patients with heterozygous familial hypercholesterolemia. Journal of Atherosclerosis and Thrombosis 2004;11(3):152‐8. [MEDLINE: ] - PubMed
Makariou 2012 {published data only}
-
- Makariou SE, Liberopoulos EN, Agouridis AP, Challa A, Elisaf M, Makariou SE, et al. Effect of rosuvastatin monotherapy and in combination with fenofibrate or omega‐3 fatty acids on serum vitamin D levels. Journal of Cardiovascular Pharmacology & Therapeutics 2012;17(4):382‐6. [MEDLINE: ] - PubMed
Marais 2008 {published data only}
-
- AstraZeneca. A 30‐week, forced‐titration and randomised, crossover, multicentre, multinational trial to evaluate the efficacy and safety of ZD4522 and atorvastatin in subjects with homozygous familial hypercholesterolaemia (4522IL/0054): Report of the first 18 weeks of treatment (Open label ZD4522 20/40/80 mg, forced‐titration period). http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐... (accessed 30 March 2001). [Study code 4522IL/0054]
-
- Marais AD, Raal FJ, Stein EA, Rader DJ, Blasetto J, Palmer M, et al. A dose‐titration and comparative study of rosuvastatin and atorvastatin in patients with homozygous familial hypercholesterolaemia. Atherosclerosis 2008;197(1):400‐6. [MEDLINE: ] - PubMed
Marino 2012 {published data only}
-
- Marino F, Maresca AM, Cosentino M, Castiglioni L, Rasini E, Mongiardi C, et al. Angiotensin II type 1 and type 2 receptor expression in circulating monocytes of diabetic and hypercholesterolemic patients over 3‐month rosuvastatin treatment. Cardiovascular Diabetology 2012;11:153. [MEDLINE: ] - PMC - PubMed
MERCURY I 2004 {published data only}
-
- AstraZeneca. An open‐label randomised, multicentre, Phase‐IIIb, parallel‐group switching study to compare the efficacy and safety of lipid‐lowering agents atorvastatin, pravastatin, simvastatin and rosuvastatin in subjects with Type IIa and IIb hypercholesterolaemia (MERCURY I). http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐... (accessed 30 March 2001). [Study code D3560C00081/4522IL/0081]
-
- Cheung RC, Morrell JM, Kallend D, Watkins C, Schuster H. Effects of switching statins on lipid and apolipoprotein ratios in the MERCURY I study. International Journal of Cardiology 2005;100(2):309‐16. [MEDLINE: ] - PubMed
-
- Schuster H, Palmer MK, Ditmarsch M. The MERCURY I open‐label extension study ‐ Subgroup analysis in patients with diabetes. British Journal of Diabetes and Vascular Disease 2008;8(3):142‐7. [EMBASE: 2008340601]
-
- Stender S, Schuster H, Barter P, Watkins C, Kallend D, MERCURY I Study Group. Comparison of rosuvastatin with atorvastatin, simvastatin and pravastatin in achieving cholesterol goals and improving plasma lipids in hypercholesterolaemic patients with or without the metabolic syndrome in the MERCURY I trial. Diabetes, Obesity & Metabolism 2005;7(4):430‐8. [MEDLINE: ] - PubMed
MERCURY II 2006 {published data only}
-
- AstraZeneca. An open label, randomized, multi‐center, Phase IIIB, parallel group switching study to compare the efficacy and safety of lipid lowering agents atorvastatin and simvastatin with rosuvastatin in high risk subjects with Type IIa and IIb hypercholesterolemia. http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐... (accessed 17 November 2004). [Study code D3560C00068/4522IL/0068]
-
- Ballantyne CM, Bertolami M, Garcia HRH, Nul D, Stein EA, Theroux P, et al. Achieving LDL cholesterol, non‐HDL cholesterol, and apolipoprotein B target levels in high‐risk patients: Measuring Effective Reductions in Cholesterol Using Rosuvastatin therapY (MERCURY) II. American Heart Journal 2006;151(5):975.e1‐975.e9. [MEDLINE: ] - PubMed
-
- Ballantyne CM, Raichlen JS, Cain VA. Statin therapy alters the relationship between apolipoprotein B and low‐density lipoprotein cholesterol and non‐high‐density lipoprotein cholesterol targets in high‐risk patients: the MERCURY II (Measuring Effective Reductions in Cholesterol Using Rosuvastatin) trial. Journal of the American College of Cardiology 2008;52(8):626‐32. [MEDLINE: ] - PubMed
-
- Izzat I. The MERCURY II trial: Benefits of rosuvastatin. British Journal of Diabetes and Vascular Disease 2006;6(4):171‐6. [EMBASE: 2006426646]
Milionis 2005 {published data only}
-
- Milionis HJ, Gazi IF, Filippatos TD, Tzovaras V, Chasiotis G, Goudevenos J, et al. Starting with rosuvastatin in primary hyperlipidemia ‐ Is there more than lipid lowering?. Angiology 2005;56(5):585‐92. [MEDLINE: ] - PubMed
Mori 2013 {published data only}
-
- Mori H, Okada Y, Tanaka Y. Effects of pravastatin, atorvastatin, and rosuvastatin in patients with type 2 diabetes mellitus and hypercholesterolemia. Diabetology International 2013;4(2):117‐25.
Moutzouri 2011 {published data only}
-
- Moutzouri E, Liberopoulos E, Mikhailidis DP, Kostapanos MS, Kei AA, Milionis H, et al. Comparison of the effects of simvastatin vs. rosuvastatin vs. simvastatin/ezetimibe on parameters of insulin resistance. International Journal of Clinical Practice 2011;65(11):1141‐8. [MEDLINE: ] - PubMed
Olsson 2001 {published data only}
-
- AstraZeneca. A Randomised, Double‐blind, Parallel‐group, Dose‐response Study with the HMG‐CoA Reductase Inhibitor ZD4522 in Subjects with Primary Hypercholesterolaemia. http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐... (accessed 28 March 2000). [Code study 4522IL/0023]
-
- AstraZeneca. A Randomised, Parallel‐Group Dose‐Response Study with the HMG‐CoA Reductase Inhibitor ZD4522 and Atorvastatin in Subjects with Primary Hypercholesterolaemia. http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐... (accessed 14 January 2000). [Code study 4522IL/0008]
-
- Olsson A G. A new statin: a new standard. The American journal of managed care. United States, 2001; Vol. 7, issue 5 Suppl:S152‐4. [MEDLINE: ] - PubMed
-
- Olsson AG, Pears J, McKellar J, Mizan J, Raza A. Effect of rosuvastatin on low‐density lipoprotein cholesterol in patients with hypercholesterolemia. American Journal of Cardiology 2001;88(5):504‐8. [MEDLINE: ] - PubMed
Olsson 2002 {published data only}
-
- AstraZeneca. A randomised, double‐blind, multinational, multicentre trial to compare the short‐term and long‐term efficacy and safety of ZD4522 and atorvastatin in the treatment of subjects with hypercholesterolaemia. http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐... (accessed 14 March 2001). [Code study 4522IL/0026]
-
- Olsson AG, Istad H, Luurila O, Ose L, Stender S, Tuomilehto J, et al. Effects of rosuvastatin and atorvastatin compared over 52 weeks of treatment in patients with hypercholesterolemia. American Heart Journal 2002;144(6):1044‐51. [MEDLINE: ] - PubMed
Paoletti 2009 {published data only}
-
- Blasetto JW, Stein EA, Brown WV, Chitra R, Raza A. Efficacy of rosuvastatin compared with other statins at selected starting doses in hypercholesterolemic patients and in special population groups. American Jjournal of Cardiology 2003;91(5A):3C‐10C. [MEDLINE: ] - PubMed
-
- Chapman MJ, Caslake M. Non‐high‐density lipoprotein cholesterol as a risk factor: Addressing risk associated with apolipoprotein B‐containing lipoproteins. European Heart Journal, Supplement 2004;6(A):A43‐A48. [EMBASE: 2004139456]
-
- Paoletti R, Fahmy M, Mahla G, Mizan J, Southworth H. Rosuvastatin demonstrates greater reduction of low‐density lipoprotein cholesterol compared with pravastatin and simvastatin in hypercholesterolaemic patients: A randomized, double‐blind study. Journal of Cardiovascular Risk 2001;8(6):383‐90. [MEDLINE: ] - PubMed
Park 2010 {published data only}
-
- ClinicalTrials.gov. A 6‐week, randomised, open‐label, parallel group, multi‐centre study to compare the efficacy of rosuvastatin 10mg with atorvastatin 10mg in the treatment of non‐diabetic metabolic syndrome subjects with raised LDL‐C. http://clinicaltrials.gov/show/NCT00335699 (accessed 25 November 2012). [Study code NCT00335699/D3560L00053 or KREST]
Patel 2011 {published data only}
-
- Patel K, Jhaveri R, George J, Qiang G, Kenedi C, Brown K, et al. Open‐label, ascending dose, prospective cohort study evaluating the antiviral efficacy of Rosuvastatin therapy in serum and lipid fractions in patients with chronic hepatitis C. Journal of Viral Hepatitis 2011;18(5):331‐7. [EMBASE: 2011197820] - PMC - PubMed
Pirro 2007 {published data only}
-
- Pirro M, Schillaci G, Mannarino MR, Savarese G, Vaudo G, Siepi D, et al. Effects of rosuvastatin on 3‐nitrotyrosine and aortic stiffness in hypercholesterolemia. Nutrition, Metabolism and Cardiovascular Diseases 2007;17(6):436‐41. [MEDLINE: ] - PubMed
Pirro 2009 {published data only}
-
- Pirro M, Schillaci G, Romagno PF, Mannarino MR, Bagaglia F, Razzi R, et al. Influence of short‐term rosuvastatin therapy on endothelial progenitor cells and endothelial function. Journal of Cardiovascular Pharmacology and Therapeutics 2009;14(1):14‐21. [MEDLINE: ] - PubMed
PLUTO 2010 {published data only}
-
- AstraZeneca. A phase IIIb, efficacy, and safety study of rosuvastatin in children and adolescents 10 to 17 years of age with heterozygous familial hypercholesterolemia (HeFH): a 12‐week, double‐blind, randomized, multicenter, placebo‐controlled study with a 40‐week, open‐label, follow‐up period. http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐... (accessed 11 March 2008). [Study code D3561C00087/4522IL/0087]
-
- Avis HJ, Hargreaves IP, Ruiter JPN, Land JM, Wanders RJ, Wijburg FA. Rosuvastatin lowers coenzyme Q10 levels, but not mitochondrial adenosine triphosphate synthesis, in children with familial hypercholesterolemia. Journal of Pediatrics. United States: Department of Pediatrics, Academic Medical Center, Amsterdam, The Netherlands., 2011; Vol. 158, issue 3:458‐62. [MEDLINE: ] - PubMed
-
- Avis HJ, Hutten BA, Gagne C, Langslet G, McCrindle BW, Wiegman A, et al. Efficacy and safety of rosuvastatin therapy for children with familial hypercholesterolemia. Journal of the American College of Cardiology 2010;55(11):1121‐6. [MEDLINE: ] - PubMed
Polenova 2009 {published data only}
-
- Polenova NV, Vaulin NA, Masenko VP, Iavelov IS, Gratsianskii NA. Rosuvastatin and fenofibrate in patients with diabetes and low high density lipoprotein cholesterol: comparison of changes of lipid levels and some markers of inflammation. Kardiologiia 2009;49(2):9‐14. [MEDLINE: ] - PubMed
-
- Semenova AE, Sergienko IV, Masenko VP, Gabrusenko SA, Kukharchuk VV, Belenkov IuN, et al. Effect of rosuvastatin therapy and myocardial revascularization on angiogenesis in coronary artery disease patients. Kardiologiia 2007;47(11):4‐8. [MEDLINE: ] - PubMed
-
- Sergienko IV, Samoilenko EI, Masenko VP, Ezhov MV, Sumarokov AB, Tkachev GA, et al. Effect of therapy with rosuvastatin on lipid spectrum, factors of inflammation and endothelial function in patients with ischemic heart disease. Kardiologiia 2006;46(5):4‐8. [MEDLINE: ] - PubMed
Postadzhiyan 2008 {published data only}
-
- Postadzhiyan AS, Tzontcheva AV, Kehajov I, Kyurkchiev S, Apostolova MD, Finkov B. Effect of conventional and more aggressive rosuvastatin treatment on markers of endothelial activation. Journal of Medical Biochemistry 2008;27(4):432‐8. [EMBASE: 2008553110]
PULSAR 2006 {published data only}
-
- AstraZeneca. A 6‐week open‐label, randomised, multicentre, Phase IIIb, parallel‐group study to compare the efficacy and safety of rosuvastatin (10 mg) with atorvastatin (20 mg) in subjects with hypercholesterolaemia and either a history of CHD or clinical evidence of CHD. Protocol D3569C00001 or 4522IL/0102 2005. [Study code D3569C0001/4522IL/0102]
-
- Clearfield MB, Amerena J, Bassand JP Hernandez Garcia HR, Miller SS, et al. Comparison of the efficacy and safety of rosuvastatin 10 mg and atorvastatin 20 mg in high‐risk patients with hypercholesterolemia ‐ Prospective study to evaluate the Use of Low doses of the Statins Atorvastatin and Rosuvastatin (PULSAR). Trials 2006;7:Article 35. [EMBASE: 2007065270] - PMC - PubMed
RADAR 2005 {published data only}
-
- Bergheanu SC, Reijmers T, Zwinderman AH, Bobeldijk I, Ramaker R, Liem AH, et al. Lipidomic approach to evaluate rosuvastatin and atorvastatin at various dosages: investigating differential effects among statins. Current Medical Research and Opinion. England: Leiden University Medical Center, Leiden, The Netherlands., 2008; Vol. 24, issue 9:2477‐87. [MEDLINE: ] - PubMed
-
- Bergheanu SC, Tol A, Dallinga‐Thie GM, Liem A, Dunselman PHJ, Bom JG, et al. Effect of rosuvastatin versus atorvastatin treatment on paraoxonase‐1 activity in men with established cardiovascular disease and a low HDL‐cholesterol. Current Medical Research and Opinion. England: Leiden University Medical Center, Leiden, The Netherlands., 2007; Vol. 23, issue 9:2235‐40. [MEDLINE: ] - PubMed
-
- Jukema JW, Liem A‐H, Dunselman PHJM, Sloot JAP, Lok DJA, Zwinderman AH. LDL‐C/HDL‐C ratio in subjects with cardiovascular disease and a low HDL‐C: Results of the RADAR (Rosuvastatin and Atorvastatin in different Dosages and Reverse cholesterol transport) study. Current Medical Research and Opinion 2005;21(11):1865‐74. [MEDLINE: ] - PubMed
-
- Karalis IK, Bergheanu SC, Wolterbeek R, Dallinga‐Thie G M, Hattori H, Tol A, et al. Effect of increasing doses of Rosuvastatin and Atorvastatin on apolipoproteins, enzymes and lipid transfer proteins involved in lipoprotein metabolism and inflammatory parameters. Current Medical Research and Opinion. England: Leiden University Medical Center, Leiden, The Netherlands., 2010; Vol. 26, issue 10:2301‐13. [MEDLINE: ] - PubMed
Raza 2000 {published data only}
-
- Raza A, inventor. Use of Cholesterol‐lowering agent. World patent 20000458/9 2000 Aug 10. [Patent Number WO20000458/9]
ROMEO 2009 {published data only}
-
- AstraZeneca. A 6‐week, randomised, open‐label, parallel group, multi‐centre study to compare the efficacy of rosuvastatin 10mg with atorvastatin 10mg in the treatment of metabolic syndrome subjects with raised LDL‐C; ROsuvastatin in MEtabolic syndrOm (ROMEO). http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐... (accessed 10 March 2009). [Study code D3560L00061]
-
- ClinicalTrials.gov. A 6‐week, randomised, open‐label, parallel group, multi‐centre study to compare the efficacy of rosuvastatin 10mg with atorvastatin 10mg in the treatment of metabolic syndrome subjects with raised LDL‐C. http://clinicaltrials.gov/ct2/show/results/NCT00395486 (accessed 30 June 2011). [Study code NCT00395486]
Rosenson 2011 {published data only}
-
- ClinicalTrials.gov. A multicenter study comparing the safety and efficacy of ABT‐335 and rosuvastatin calcium combination therapy to monotherapy in subjects with dyslipidemia. http://clinicaltrials.gov/ct2/show/NCT00463606 (accessed 27 September 2012). [Study code NCT00463606]
-
- ClinicalTrials.gov. Evaluate safety and efficacy of ABT‐335 in combination with rosuvastatin calcium in subjects with multiple abnormal lipid levels in the blood. http://clinicaltrials.gov/show/NCT00300482 (assessed 03 June 2009). [Study code NCT00300482]
Saito 2003 {published data only}
-
- AstraZeneca. A randomised, double‐blind, dose‐response study with the HMG‐CoA reductase inhibitor ZD4522 in subjects with hyperlipidaemis (a Phase II clinical study). http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐... (accessed 10 December 2001). [Study code 4522IL/0055]
-
- Saito Y. Randomized dose‐response study of rosuvastatin in Japanese patients with hypercholesterolemia. Journal of Atherosclerosis and Thrombosis 2003;10(6):329. [MEDLINE: ] - PubMed
Saito 2007 {published data only}
-
- Saito Y, Yamada N, Shirai K, Sasaki J, Ebihara Y, Yanase T, et al. Effect of rosuvastatin 5‐20 mg on triglycerides and other lipid parameters in Japanese patients with hypertriglyceridemia. Atherosclerosis 2007;194(2):505‐11. [EMBASE: 2007483718] - PubMed
Schneck 2003 {published data only}
-
- AstraZeneca Pharmaceuticals. A 6‐week, randomized, double‐blind, multicenter trial to evaluate the safety and efficacy of ZD4522 (5, 10, 20, 40, and 80 mg) and atorvastatin (10, 20, 40, and 80 mg) across their respective dose ranges in the treatment of subjects with hypercholesterolemia. http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐... (accessed 06 December 2000). [Study code 4522IL/0033]
-
- Packard CJ. Apolipoproteins: The new prognostic indicator?. European Heart Journal, Supplement 2003;5(D):D9‐D16. [EMBASE: 2003238419]
-
- Schneck DW, Knopp RH, Ballantyne CM, McPherson R, Chitra RR, Simonson SG. Comparative effects of rosuvastatin and atorvastatin across their dose ranges in patients with hypercholesterolemia and without active arterial disease. American Journal of Cardiology 2003;91(1):33‐41. [MEDLINE: ] - PubMed
Schwartz 2004 {published data only}
-
- AstraZeneca. A 24‐week, randomized, double‐blind, multicenter trial to evaluate the efficacy and safety of starting and maximum doses of ZD4522 and atorvastatin in the treatment of high risk hypercholesterolemic subjects. http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐... (accessed 23 March 2001). [Study code 4522IL/0025]
-
- Schwartz GG, Bolognese MA, Tremblay BP Caplan R, Hutchinson H, Raza A, et al. Efficacy and safety of rosuvastatin and atorvastatin in patients with hypercholesterolemia and a high risk of coronary heart disease: a randomized, controlled trial. American Heart Journal 2004;148(1):e4. [MEDLINE: ] - PubMed
Semenova 2009 {published data only}
-
- Semenova AE, Sergienko IV, Masenko VP, Ezhov MV, Gabrusenko SA, Kuharchuk VV, et al. The influence of rosuvastatin therapy and percutaneous coronary intervention on angiogenic growth factors in coronary artery disease patients. Acta Cardiologica 2009;64(3):405‐9. [MEDLINE: ] - PubMed
-
- Semenova AE, Sergienko IV, Masenko VP, Gabrusenko SA, Kukharchuk VV, Belenkov IuN. Effect of rosuvastatin therapy and myocardial revascularization on angiogenesis in coronary artery disease patients. Kardiologiia. Russia (Federation): Cardiology Research Complex, ul Tretiya Cherepkovskaya 15a, Moscow, Russia., 2007; Vol. 47, issue 11:4‐8. [MEDLINE: ] - PubMed
Shepherd 2004 {published data only}
-
- AstraZeneca. An 18‐week, randomised, double‐blind, multicentre, placebo‐controlled trial to evaluate the efficacy and safety of rosuvastatin (5 and 10 mg) in the treatment of hypercholesterolaemic postmenopausal women receiving hormone replacement therapy. http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐... (accessed 24 August 2001). [Study code 4522IL/0032]
-
- Shepherd J. Lipid‐modifying effects of rosuvastatin in postmenopausal women with hypercholesterolemia who are receiving hormone replacement therapy. Current Medical Research and Opinion 2004;20(10):1571. [MEDLINE: ] - PubMed
SHUKRA 2009 {published data only}
-
- ClinicalTrials.com. Study of Asian patients with hypercholesterolaemia in the UK ‐ rosuvastatin 5mg versus atorvastatin 10mg. http://clinicaltrials.gov/show/NCT00427960 (accessed 30 November 2010). [Study code NCT00427960]
Siddiqi 2013 {published data only}
SOLAR 2007 {published data only}
-
- AstraZeneca. A 12‐week, randomized, open‐label, 3 arm parallel group, multicenter, Phase IIIb study comparing the efficacy and safety of rosuvastatin with atorvastatin and simvastatin achieving NCEP ATP III LDL‐C goals in high risk subjects with hypercholesterolaemia in the managed care setting. http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐... (accessed 21 July 2005). [Study code D3560L00023]
-
- Insull. Erratum: Achieving low‐density lipoprotein cholesterol goals in high‐risk patients in managed care: Comparison of rosuvastatin, atorvastatin, and simvastatin in the SOLAR trial (Mayo Clinic Proceedings (2007) 82, (543‐550)). Mayo Clinic Proceedings 2007;82(7):890. [EMBASE: 2007319758] - PubMed
-
- Insull W Jr, Ghali JK, Hassman DR, Y As JW, Gandhi SK, Miller E, et al. SOLAR Study Group. Achieving low‐density lipoprotein cholesterol goals in high‐risk patients in managed care: comparison of rosuvastatin, atorvastatin, and simvastatin in the SOLAR trial.[Erratum appears in Mayo Clin Proc. 2007 Jul;82(7):890]. Mayo Clinic Proceedings 2007;82(5):543‐50. [MEDLINE: ] - PubMed
STARSHIP 2006 {published data only}
-
- AstraZeneca. A 6‐week, randomized, open‐label, comparative study to evaluate the efficacy and safety of rosuvastatin and atorvastatin in the treatment of hypercholesterolaemia in Hispanic subjects. http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐... (accessed 22 December 2005). [Study code D3560L00027_OLE]
-
- Lloret R, Ycas J, Stein M, Haffner S, STARSHIP Study Group. Comparison of rosuvastatin versus atorvastatin in Hispanic‐Americans with hypercholesterolemia (from the STARSHIP trial). American Journal of Cardiology 2006;98(6):768‐73. [MEDLINE: ] - PubMed
Stein 2007a {published data only}
-
- Stein EA, Marais AD, Ducobu J, Farnier M, Gavish D, Hauner H, et al. Comparison of short‐term renal effects and efficacy of rosuvastatin 40 mg and simvastatin 80 mg, followed by assessment of long‐term renal effects of rosuvastatin 40 mg, in patients with dyslipidemia. Journal of Clinical Lipidology 2007;1(4):287‐99. [EMBASE: 2007438964] - PubMed
Stein 2007b {published data only}
-
- AstraZeneca. A 48‐week open‐label, non‐comparative, multicentre, Phase IIIb study to evaluate the efficacy and safety of the lipid‐regulating agent rosuvastatinin the treatment of patients with Fredrickson Type IIa and Type IIb dyslipidaemia, including heterozygous familial hypercholesterolaemia. http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐... (accessed 14 March 2005). [Study code D3560C00091]
-
- Stein EA, Amerena J, Ballantyne CM, Brice E, Farnier M, Guthrie RM, et al. Long‐term efficacy and safety of rosuvastatin 40 mg in patients with severe hypercholesterolemia. American Journal of Cardiology 2007;100(9):1387‐96. [MEDLINE: ] - PubMed
STELLAR 2003 {published data only}
-
- Ai M, Otokozawa S, Asztalos BF, Nakajima K, Stein E, Jones PH, et al. Effects of maximal doses of atorvastatin versus rosuvastatin on small dense low‐density lipoprotein cholesterol levels. American Journal of Cardiology 2008; Vol. 101, issue 3:315‐8. [MEDLINE: ] - PubMed
-
- Asztalos BF, Maulf F, Dallal GE, Stein E, Jones PH, Horvath KV, et al. Comparison of the effects of high doses of rosuvastatin versus atorvastatin on the subpopulations of high‐density lipoproteins. American Journal of Cardiology. United States: Cardiovascular Research Laboratory, Tufts University, Boston, Massachusetts, USA. bela.asztalos@tufts.edu, 2007; Vol. 99, issue 5:681‐5. [MEDLINE: ] - PubMed
-
- Barrios V, Lobos JM, Serrano A, Brosa M, Capel M, Alvarez Sanz C, et al. Cost‐effectiveness analysis of rosuvastatin vs generic atorvastatin in Spain. Journal of Medical Economics 2012;15 Suppl 1:45‐54. [MEDLINE: ] - PubMed
-
- Chong PH, Varner D. Cost‐efficacy analysis of 3‐hydroxy‐3‐methylglutaryl coenzyme a reductase inhibitors based on results of the STELLAR trial: clinical implications for therapeutic selection. Pharmacotherapy. United States: Department of Pharmacy Practice, College of Pharmacy, University of Illinois at Chicago, Chicago, Illinois, USA., 2005; Vol. 25, issue 2:270‐8. [MEDLINE: ] - PubMed
-
- ClinicalTrials.gov. A 6 week open label, dose comparison study to evaluate the safety and efficacy of rosuvastatin versus atorvastatin, pravastatin, and simvastatin in subjects with hypercholesterolemia. http://clinicaltrials.gov/show/NCT00654537 (accessed 13 March 2009). [Study code NCT00654537]
Szapary 2012 {published data only}
-
- Szapary L, Feher G. Effectiveness of generic rosuvastatin in patients with ischaemic cerebrovascular disease. Orvosi Hetilap. Medicina Kiado RT (Zoltan u. 8., Budapest 1054, Hungary), 2012; Vol. 153, issue 22:857‐60. [MEDLINE: ] - PubMed
Takebayashi 2009 {published data only}
-
- Takebayashi K, Suetsugu M, Matsumoto S, Aso Y, Inukai T. Effects of rosuvastatin and colestimide on metabolic parameters and urinary monocyte chemoattractant protein‐1 in type 2 diabetic patients with hyperlipidemia. Southern Medical Journal 2009;102(4):361‐8. [MEDLINE: ] - PubMed
Tateishi 2011 {published data only}
-
- Tateishi J. Efficacy of three potent statins in patients with hypercholesterolemia who were newly prescribed statins. Therapeutic Research 2011;32(12):1653‐61. [EMBASE: 2012051834]
Tsunoda 2011 {published data only}
-
- Tsunoda S. Efficacy and safety of rosuvastatin 2.5 mg and atorvastatin 10 mg in patients with hypercholesterolemia. Therapeutic Research 2011;32(11):1507‐12. [EMBASE: 2011711049]
Wang 2012 {published data only}
-
- Wang Guo Hua, Zhang Xu, Cao Juan, Li Hai Tao, Yin Di, Zhou Chang Ju. Lipid‐lowering efficacy and safety of rosuvastatin calcium in treatment of patients with chronic kidney disease. Guangdong Yixue 2012;33(1):125‐7. [CAPLUS AN 2012:1577414]
Weinstein 2013 {published data only}
-
- Weinstein DL, Williams LA, Carlson DM, Kelly MT, Burns KM, Setze CM, et al. A randomized, double‐blind study of fenofibric Acid plus rosuvastatin compared with rosuvastatin alone in stage 3 chronic kidney disease. Clinical Therapeutics 2013;35(8):1186‐98. - PubMed
Wongwiwatthananukit 2006 {published data only}
-
- Wongwiwatthananukit S, Sansanayudh N, Dhummauppakorn R, Kitiyadisai C. Efficacy and safety of rosuvastatin every other day compared with once daily in patients with hypercholesterolemia. Annals of Pharmacotherapy 2006;40(11):1917‐23. [MEDLINE: ] - PubMed
Yamamoto 2002 {published data only}
-
- Yamamoto A, Arakawa K, Sasaki J, Matsuzawa Y, Takemura K, Tsushima M, et al. Clinical effects of rosuvastatin, a new HMG‐CoA reductase inhibitor, in Japanese patients with primary hypercholesterolemia: an early phase II study. Journal of Atherosclerosis and Thrombosis 2002;9(1):48‐56. [MEDLINE: ] - PubMed
Yanagi 2011 {published data only}
-
- Yanagi K, Monden T, Ikeda S, Matsumura M, Kasai K. A crossover study of rosuvastatin and pitavastatin in patients with type 2 diabetes. Advances in Therapy 2011;28(2):160‐71. [MEDLINE: ] - PubMed
Yoshino 2012 {published data only}
-
- Yoshino G, Nakano S, Matsumoto T, Murakami E, Morita T, Kuboki K. Rosuvastatin reduces plasma small dense LDL‐cholesterol predominantly in non‐diabetic hypercholesterolemic patients. Pharmacology & Pharmacy 2012;3(1):72‐8. [CAPLUS AN 2012:748036]
References to studies excluded from this review
AstraZeneca 2007 {published data only}
-
- AstraZeneca. A multicenter, randomized, double‐blind, parallel‐group, dose titration ( 10 mg and 20 mg) study to compare the efficacy and safety of Rosuvastatin versus Atorvastatin in patients with primary hypercholesterolemia. http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐... (accessed 18 January 2007). [Study code 4522SP/0001]
Bays 2011 {published data only}
-
- Bays HE, Davidson MH, Massaad R, Flaim D, Lowe RS, Tershakovec AM, et al. Safety and efficacy of ezetimibe added on to rosuvastatin 5 or 10 mg versus up‐titration of rosuvastatin in patients with hypercholesterolemia (the ACTE Study). American Journal of Cardiology 2011;108(4):523‐30. [MEDLINE: ] - PubMed
Bertolotti 2012 {published data only}
-
- Bertolotti M, Puppo M, Corna F, Anzivino C, Gabbi C, Baldelli E, et al. Increased appearance rate of 27‐hydroxycholesterol in vivo in hypercholesterolemia: a possible compensatory mechanism. Nutrition Metabolism & Cardiovascular Diseases 2012;22(10):823‐30. [MEDLINE: ] - PubMed
Bottaro 2008 {published data only}
-
- Bottaro EG, Caravello O, Scapellato PG, Stambulian M, Vidal GI, Loggia V, et al. Rosuvastatin for the treatment of dyslipidemia in HIV‐infected patients receiving highly active antiretroviral therapy. Preliminary experience]. Spanish]. Enfermedades Infecciosas y Microbiologia Clinica 2008;26(6):325‐9. [MEDLINE: ] - PubMed
Burmeister 2009 {published data only}
-
- Burmeister JE, Mittersteiner DR, Campos BM. Rosuvastatin in hemodialysis: Short‐term effects on lipids and C‐reactive protein. Journal of Nephrology 2009;22(1):83‐9. [MEDLINE: ] - PubMed
Calza 2008 {published data only}
-
- Calza L, Manfredi R, Colangeli V, Pocaterra D, Pavoni M, Chiodo F. Rosuvastatin, pravastatin, and atorvastatin for the treatment of hyper‐cholesterolaemia in HIV‐infected patients receiving protease inhibitors. Current HIV Research. Bentham Science Publishers B.V. (P.O. Box 294, Bussum 1400 AG, Netherlands), 2008; Vol. 6, issue 6:572‐8. [EMBASE: 2009079366] - PubMed
Calza 2012 {published data only}
-
- Calza L, Trapani F, Bartoletti M, Manfredi R, Colangeli V, Borderi M, et al. Statin therapy decreases serum levels of high‐sensitivity C‐reactive protein and tumor necrosis factor‐ in HIV‐infected patients treated with ritonavir‐boosted protease inhibitors. HIV Clinical Trials 2012;13(3):153‐61. [MEDLINE: ] - PubMed
Calza 2013 {published data only}
-
- Calza L, Manfredi R, Colangeli V, Trapani FF, Salvadori C, Magistrelli E, et al. Two‐year treatment with rosuvastatin reduces carotid intima‐media thickness in HIV type 1‐infected patients receiving highly active antiretroviral therapy with asymptomatic atherosclerosis and moderate cardiovascular risk. AIDS Research & Human Retroviruses 2013;29(3):547‐56. [MEDLINE: ] - PubMed
COMPELL 2007 {published data only}
-
- Kos Pharmaceuticals. Comparative efficacy evaluation of lipids when treated with niaspan & statin or other lipid‐modifying therapies‐COMPELL. http://clinical‐trials.findthedata.org/l/43529/Comparative‐Efficacy‐Evaluation‐of‐Lipids... 2006. [Study code NCT00079638]
-
- McKenney JM, Jones PH, Bays HE, Knopp RH, Kashyap ML, Ruoff GE, et al. Comparative effects on lipid levels of combination therapy with a statin and extended‐release niacin or ezetimibe versus a statin alone (the COMPELL study). Atherosclerosis 2007;192(2):432‐7. [MEDLINE: ] - PubMed
DISCOVERY‐Alpha 2006 {published data only}
-
- Binbrek AS, Elis A. Rosuvastatin versus atorvastatin in achieving lipid goals in patients at high risk for cardiovascular disease in clinical practice: A randomized, open‐label, parallel‐group, multicenter study (DISCOVERY Alpha study). Current Therapeutic Research ‐ Clinical and Experimental 2006;67(1):21. [EMBASE: 2006376830] - PMC - PubMed
Domingos 2012 {published data only}
-
- Domingos H, Cunha RV, Paniago AM, Souza AS, Rodrigues RL, Domingos JA, et al. Rosuvastatin and ciprofibrate in the treatment of dyslipidemia in patients with HIV. Arquivos Brasileiros de Cardiologia 2012;99(5):997‐1007. [MEDLINE: ] - PubMed
Fonseca 2005 {published data only}
-
- Fonseca FAH, Ruiz A, Cardona‐Munoz EG, Silva JM, Fuenmayor N, Marotti M, et al. The DISCOVERY PENTA study: a DIrect Statin COmparison of LDL‐C Value‐‐an Evaluation of Rosuvastatin therapY compared with atorvastatin. Current Medical Research and Opinion 2005;21(8):1307‐15. [MEDLINE: ] - PubMed
Gadarla 2008 {published data only}
-
- Gadarla M, Kearns AK, Thompson PD. Efficacy of rosuvastatin (5 mg and 10 mg) twice a week in patients intolerant to daily statins. American Journal of Cardiology 2008;101(12):1747‐8. [MEDLINE: ] - PubMed
Gliozzi 2013 {published data only}
-
- Gliozzi M, Walker R, Muscoli S, Vitale C, Gratteri S, Carresi C, et al. Bergamot polyphenolic fraction enhances rosuvastatin‐induced effect on LDL‐cholesterol, LOX‐1 expression and protein kinase B phosphorylation in patients with hyperlipidemia. International journal of cardiology 2013;170:140‐5. - PubMed
Goldberg 2011 {published data only}
-
- Goldberg AC, Bittner V, Pepine CJ, Kelly MT, Thakker K, Setze CM, et al. Efficacy of fenofibric acid plus statins on multiple lipid parameters and its safety in women with mixed dyslipidemia. American Journal of Cardiology 2011;107(6):898‐905. [MEDLINE: ] - PubMed
Johns 2007 {published data only}
Jyoti 2008 {published data only}
-
- Jyoti N. Comparative evaluation of atorvastatin and rosuvastatin in patients of dyslipidemia. JK Practitioner 2008;15(1‐4):27‐35. [EMBASE: 2009190395]
Katabami 2014 {published data only}
-
- Katabami T, Murakami M, Kobayashi S, Matsui T, Ujihara M, Takagi S, et al. Efficacy of low‐dose rosuvastatin in patients with type 2 diabetes and hypo high‐density lipoprotein cholesterolaemia. Journal of International Medical Research 2014;42:457‐67. - PubMed
Khan 2014 {published data only}
-
- Khan M A, Murti K, Grover V, Lal K, Singh D, Das P, et al. Atorvastatin vs rosuvastatin; Fenofibrate as an add on: An exploratory study. International Journal of Pharmacy and Pharmaceutical Sciences 2014;6:493‐8.
Kiser 2008 {published data only}
-
- Kiser JJ, Gerber JG, Predhomme JA, Wolfe P, Flynn DM, Hoody DW. Drug/drug interaction between lopinavir/ritonavir and rosuvastatin in healthy volunteers. Journal of Acquired Immune Deficiency Syndromes 2008; Vol. 47, issue 5:570‐8. [MEDLINE: ] - PubMed
Li 2012a {published data only}
-
- Li R‐F, Yi G‐Z, Liu Z‐H. Efficacy of rosuvastatin in the treatment of early diabetic nephropathy. Chinese Journal of New Drugs. Chinese Journal of New Drugs Co. Ltd. (Floor 8. Lunyang Building No.6 Beisanhuan Zhonglu, Xicheng District, Beijing 100120, China), 2012; Vol. 21, issue 3:298. [EMBASE: 2012227641]
Li 2012b {published data only}
-
- Li Bo, Liu Wei Wei, Wo Jin Shan. Effects of rosuvastatin on apolipoprotein b/apolipoprotein a1 in patients with metabolic syndrome. Shiyong Yixue Zazhi 2012;28(7):1181‐3. [CENTRAL: CN‐00866854 NEW]
Polis 2009 {published data only}
-
- Polis AB, Abate N, Catapano AL, Ballantyne CM, Davidson MH, Smugar SS, et al. Low‐density lipoprotein cholesterol reduction and goal achievement with ezetimibe/simvastatin versus atorvastatin or rosuvastatin in patients with diabetes, metabolic syndrome, or neither disease, stratified by National Cholesterol Education Program risk category. Metabolic Syndrome and Related Disorders 2009;7(6):601‐10. [MEDLINE: ] - PubMed
Puccetti 2011 {published data only}
-
- Puccetti L, Santilli F, Pasqui AL, Lattanzio S, Liani R, Ciani F, et al. Effects of atorvastatin and rosuvastatin on thromboxane‐dependent platelet activation and oxidative stress in hypercholesterolemia. Atherosclerosis 2011;214(1):122‐8. [MEDLINE: ] - PubMed
Riccioni 2012 {published data only}
-
- Riccioni G, Scotti L, Ilio E, Bucciarelli V, D'Orazio N, Aceto A, et al. Rosuvastatin effect on intima media thickness in adult vs elderly patients. Frontiers in Bioscience 2012;4:2718‐21. [MEDLINE: ] - PubMed
Rossi 2009 {published data only}
-
- Rossi M, Carpi A, Maria C, Franzoni F, Galetta F, Santoro G. Skin blood flow motion and microvascular reactivity investigation in hypercholesterolemic patients without clinically manifest arterial diseases. Physiological Research / Academia Scientiarum Bohemoslovaca 2009;58(1):39‐47. [MEDLINE: ] - PubMed
Roth 2010 {published data only}
-
- Roth EM, McKenney JM, Kelly MT, Setze CM, Carlson DM, Gold A, et al. Efficacy and safety of rosuvastatin and fenofibric acid combination therapy versus simvastatin monotherapy in patients with hypercholesterolemia and hypertriglyceridemia: a randomized, double‐blind study. American Journal of Cardiovascular Drugs : drugs, devices, and other interventions 2010;10(3):175‐86. [MEDLINE: ] - PubMed
Talavera 2013 {published data only}
-
- Talavera JO, Martinez G, Cervantes JL, Marin JA, Rodriguez‐Briones I, Gonzalez JG, et al. A double‐blind, double‐dummy, randomized, placebo‐controlled trial to evaluate the effect of statin therapy on triglyceride levels in Mexican hypertriglyceridemic patients. Current Medical Research & Opinion 2013;29(4):379‐86. [MEDLINE: ] - PubMed
Van Der Lee, 2007 {published data only}
-
- Lee, Sankatsing R, Schippers E, Vogel M, Fatkenheuer G, V, et al. Pharmacokinetics and pharmacodynamics of combined use of lopinavir/ritonavir and rosuvastatin in HIV‐infected patients. Antiviral Therapy 2007;12(7):1127‐32. [MEDLINE: ] - PubMed
Van Der Lee, 2008 {published data only}
-
- Lee, Sankatsing R, Schippers E, Vogel M, Rockstroh J, Fatkenheuer G, et al. Pharmacokinetics and pharmacodynamics of simultaneous use of lopinavir + ritonavir and rosuvastatin. Pharmaceutisch Weekblad 2008;143(7):42‐5. [EMBASE: 2008103375]
Yun 2012 {published data only}
-
- Yun KH, Shin SN, Ko JS, Rhee SJ, Kim NH, Oh SK, et al. Rosuvastatin‐induced high‐density lipoprotein changes in patients who underwent percutaneous coronary intervention for non‐ST‐segment elevation acute coronary syndrome. Journal of Cardiology 2012;60(5):383‐8. [MEDLINE: ] - PubMed
References to studies awaiting assessment
Abid 2013 {published data only}
-
- Abid Shah M, Suleman S, Hussain Munir A. Comparative efficacy and safety profile of 5 MG rosuvastatin versus 10 MG rosuvastatin in patients with ischemic heart disease. Journal of Medical Sciences (Peshawar) 2013;21:35‐9.
Anagnostis 2014 {published data only}
-
- Anagnostis P, Adamidou F, Slavakis A, Polyzos SA, Selalmatzidou D, Panagiotou A, et al. Comparative effect of atorvastatin and rosuvastatin on 25‐hydroxy‐Vitamin D levels in non‐diabetic patients with Dyslipidaemia: A prospective randomized open‐label pilot study. Open Cardiovascular Medicine Journal 2014;8:55‐60. - PMC - PubMed
Arshad 2014 {published data only}
Ballantyne 2014 {published data only}
-
- Ballantyne CM, Hoogeveen RC, Raya JL, Cain VA, Palmer MK, Karlson BW, et al. Efficacy, safety and effect on biomarkers related to cholesterol and lipoprotein metabolism of rosuvastatin 10 or 20 mg plus ezetimibe 10 mg vs. simvastatin 40 or 80 mg plus ezetimibe 10 mg in high‐risk patients: Results of the GRAVITY randomized study. Atherosclerosis 2014;232:86‐93. - PubMed
Capoulade 2013 {published data only}
-
- Capoulade R, Clavel MA, Dumesnil JG, Chan KL, Teo KK, Tam JW, et al. Insulin resistance and LVH progression in patients with calcific aortic stenosis: A substudy of the ASTRONOMER trial. JACC: Cardiovascular Imaging 2013;6:165‐74. - PubMed
Capoulade 2014 {published data only}
-
- Capoulade R, Cote N, Mathieu P, Chan KL, Clavel MA, Dumesnil JG, et al. Circulating levels of matrix gla protein and progression of aortic stenosis: a substudy of the Aortic Stenosis Progression Observation: Measuring Effects of rosuvastatin (ASTRONOMER) trial. Canadian Journal of Cardiology 2014;30:1088‐95. - PubMed
Daida 2014 {published data only}
-
- Daida H, Nohara R, Hata M, Kaku K, Kawamori R, Kishimoto J, et al. Can intensive lipid‐lowering therapy improve the carotid Intima‐media thickness in Japanese subjects under primary prevention for cardiovascular disease?: The JART and JART extension subanalysis. Journal of Atherosclerosis and Thrombosis 2014;21:739‐54. - PubMed
Davidson 2014 {published data only}
-
- Davidson MH, Phillips AK, Kling D, Maki KC. Addition of omega‐3 carboxylic acids to statin therapy in patients with persistent hypertriglyceridemia. Expert Review of Cardiovascular Therapy 2014;12:1045‐54. - PubMed
Deguchi 2014 {published data only}
-
- Deguchi I, Horiuchi Y, Hayashi T, Sehara Y, Kato Y, Ohe Y, et al. Effects of Rosuvastatin on Serum Lipids and Arteriosclerosis in Dyslipidemic Patients with Cerebral Infarction. Journal of Stroke & Cerebrovascular Diseases 2014;23:2007‐11. - PubMed
Florentin 2013a {published data only}
-
- Florentin M, Liberopoulos EN, Rizos CV, Kei AA, Liamis G, Kostapanos MS, et al. Colesevelam plus rosuvastatin 5mg/day versus rosuvastatin 10mg/day alone on markers of insulin resistance in patients with hypercholesterolemia and impaired fasting glucose. Metabolic Syndrome & Related Disorders 2013;11:152‐6. - PubMed
Graziano 2012 {published data only}
-
- Graziano R, Scotti L, Ilio E, Bucciarelli V, D'Orazio N, Aceto A, et al. Rosuvastatin effect on intima media thickness in adult vs elderly patients. Frontiers in Bioscience ‐ Elite 2012;4 E:2618‐21. - PubMed
Jing 2013a {published data only}
-
- Jing S, Sun NL, Li XY, Hua Q, Wang L, Li Z Q, et al. Efficacy and safety of rosuvastatin in the treatment of hypercholesterolemia. [Chinese]. Chinese Journal of New Drugs 2013; Vol. 22:937‐940+960.
Kim 2013a {published data only}
Koh 2013a {published data only}
-
- Koh KK, Quon MJ, Sakuma I, Han SH, Choi H, Lee K, et al. Differential metabolic effects of rosuvastatin and pravastatin in hypercholesterolemic patients. International Journal of Cardiology 2013;166:509‐15. - PubMed
Kouvelos 2013 {published data only}
-
- Kouvelos GN, Arnaoutoglou EM, Matsagkas MI, Kostara C, Gartzonika C, Bairaktari ET, et al. Effects of rosuvastatin with or without ezetimibe on clinical outcomes in patients undergoing elective vascular surgery: results of a pilot study. Journal of Cardiovascular Pharmacology and Therapeutics 2013;18:5‐12. - PubMed
Kurtoglu 2014 {published data only}
-
- Kurtoglu E, Balta S, Sincer I, Altas Y, Atas H, Yilmaz M, et al. Comparision of effects of rosuvastatin versus atorvastatin treatment on plasma levels of asymmetric dimethylarginine in patients with hyperlipidemia having coronary artery disease. Angiology 2014;65:788‐93. - PubMed
Lee 2013 {published data only}
-
- Lee HK, Hu M, Lui SS, Ho CS, Wong CK, Tomlinson B. Effects of polymorphisms in ABCG2, SLCO1B1, SLC10A1 and CYP2C9/19 on plasma concentrations of rosuvastatin and lipid response in Chinese patients. Pharmacogenomics 2013;14:1283‐94. - PubMed
Liberopoulos 2013a {published data only}
-
- Liberopoulos EN, Moutzouri E, Rizos CV, Barkas F, Liamis G, Elisaf MS. Effects of manidipine plus rosuvastatin versus olmesartan plus rosuvastatin on markers of insulin resistance in patients with impaired fasting glucose, hypertension, and mixed dyslipidemia. Journal of Cardiovascular Pharmacology & Therapeutics 2013;18:113‐8. - PubMed
McGuire 2014 {published data only}
-
- McGuire TR, Kalil AC, Dobesh PP, Klepser DG, Olsen KM. Anti‐inflammatory effects of rosuvastatin in healthy subjects: A prospective longitudinal study. Current Pharmaceutical Design 2014;20:1156‐60. - PubMed
Mori 2013a {published data only}
-
- Mori H, Okada Y, Tanaka Y. Effects of pravastatin, atorvastatin, and rosuvastatin in patients with type 2 diabetes mellitus and hypercholesterolemia. Diabetology International 2013; Vol. 4:117‐25.
Moutzouri 2013 {published data only}
-
- Moutzouri E, Liberopoulos EN, Tellis CC, Milionis HJ, Tselepis AD, Elisaf MS. Comparison of the effect of simvastatin versus simvastatin/ezetimibe versus rosuvastatin on markers of inflammation and oxidative stress in subjects with hypercholesterolemia. Atherosclerosis 2013;231:8‐14. - PubMed
Nohara 2013 {published data only}
-
- Nohara R, Daida H, Hata M, Kaku K, Kawamori R, Kishimoto J, et al. Effect of long‐term intensive lipid‐lowering therapy with rosuvastatin on progression of carotid intima‐media thickness‐‐Justification for Atherosclerosis Regression Treatment (JART) extension study. Circulation Journal 2013;77:1526‐33. - PubMed
Sharma 2014 {published data only}
-
- Sharma A, Joshi PH, Rinehart S, Thakker KM, Lele A, Voros S. Baseline very low‐density lipoprotein cholesterol is associated with the magnitude of triglyceride lowering on statins, fenofibric acid, or their combination in patients with mixed dyslipidemia. Journal of Cardiovascular Translational Research 2014;7:465‐74. - PubMed
Takase 2014 {published data only}
-
- Takase B, Hattori H, Tanaka Y, Nagata M, Ishihara M. Anti‐sympathetic action enhances statin's pleiotropic effects: The combined effect of rosuvastatin and atenolol on endothelial function. International Angiology 2014;33:27‐34. - PubMed
Wang 2014 {published data only}
-
- Wang P, Liu Y, Wang Z, Zhao N, Ye H, Ren L. Effects of rosuvastatin on arterial stiffness in hyperlipidemia patients. [Chinese]. National Medical Journal of China 2014;94:2452‐4. - PubMed
Weinstein 2013a {published data only}
-
- Weinstein DL, Williams LA, Carlson DM, Kelly MT, Burns KM, Setze CM, et al. A randomized, double‐blind study of fenofibric acid plus rosuvastatin compared with rosuvastatin alone in stage 3 chronic kidney disease.[Erratum appears in Clin Ther. 2013 Nov;35(11):1862]. Clinical therapeutics 2013;35:1186‐98. - PubMed
Wu 2014 {published data only}
-
- Wu H, Han YL, Wang XZ, Li Y, Xu K, Li J, et al. Effects of short‐term rosuvastatin therapy on heart and kidney function in patients with acute coronary syndrome combining diabetes mellitus and concomitant chronic kidney disease. [Chinese]. Medical Journal of Chinese People's Liberation Army 2014;39:546‐52.
Yamazaki 2013 {published data only}
-
- Yamazaki T, Nohara R, Daida H, Hata M, Kaku K, Kawamori R, et al. Intensive Lipid‐Lowering Therapy for Slowing Progression as Well as Inducing Regression of Atherosclerosis in Japanese Patients Subanalysis of the JART Study. International Heart Journal 2013;54:33‐9. - PubMed
Yogo 2014 {published data only}
-
- Yogo M, Sasaki M, Ayaori M, Kihara T, Sato H, Takiguchi S, et al. Intensive lipid lowering therapy with titrated rosuvastatin yields greater atherosclerotic aortic plaque regression: Serial magnetic resonance imaging observations from RAPID study. Atherosclerosis 2014;232:31‐9. - PubMed
Yokoi 2014 {published data only}
-
- Yokoi H, Nohara R, Daida H, Hata M, Kaku K, Kawamori R, et al. Change in carotid intima‐media thickness in a high‐risk group of patients by intensive lipid‐lowering therapy with rosuvastatin: subanalysis of the JART study. International Heart Journal 2014;55:146‐52. - PubMed
Additional references
Adams 2012a
Bandolier 2004
-
- Bandolier. Cholesterol lowering with statins. Bandolier 2004:121‐2.
Boekholdt 2012
-
- Boekholdt SM, Arsenault BJ, Mora S, Pedersen TR, LaRosa JC, Nestel PJ, Simes RJ, Durrington P, Hitman GA, Welch KM, DeMicco DA, Zwinderman AH, Clearfield MB, Downs JR, Tonkin AM, Colhoun HM, Gotto AM Jr, Ridker PM, Kastelein JJ. Association of LDL cholesterol, non–HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins: a meta‐analysis. JAMA 2012;307(12):1302‐9. [MEDLINE: ] - PubMed
CDC 2011
-
- Centers for Disease Control and Prevention (CDC). Million hearts: strategies to reduce the prevalence of leading cardiovascular disease risk factors‐‐United States, 2011. MMWR ‐ Morbidity & Mortality Weekly Report 2011;60(36):1248‐51. [MEDLINE: ] - PubMed
Choumerianou 2005
-
- Choumerianou DM, Dedoussis GV. Familial hypercholesterolemia and response to statin therapy according to LDLR genetic background. Clinical Chemistry & Laboratory Medicine. Germany: Department of Science of Dietetics‐Nutrition, Harokopio University of Athens, Greece., 2005; Vol. 43, issue 8:793‐801. [MEDLINE: ] - PubMed
Crestor Prescribing Information 2015
-
- Highlights of Prescribing Information, 2015. https://www.astrazeneca.ca/content/dam/az‐ca/downloads/productinformatio... 2015.
CTT 2005
-
- Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R, Cholesterol Treatment Trialists' (CTT) Collaborators. Efficacy and safety of cholesterol‐lowering treatment: prospective meta‐analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366(9493):1267‐78. [MEDLINE: ] - PubMed
Edwards 2003
Furukawa 2006
-
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7‐10. [MEDLINE: ] - PubMed
Gaw 2000
-
- Gaw A, Packard CJ, Shepherd J. Statins : the HMG CoA Reductase Inhibitors in Perspective. London, England: Martin Dunitz Ltd, 2000. [ISBN 1853174688]
Goodman 2011
-
- Goodman LS, Brunton LL, Chabner B, Knollmann BC. Goodman & Gilman's Pharmacological Basis of Therapeutics. 12th Edition. New York: McGraw‐Hill, 2011. [ISBN: 9780071624428]
Grundy 2004
-
- Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC Jr, Stone NJ, Coordinating Committee of the National Cholesterol Education Program. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. Journal of the American College of Cardiology 2004;44(3):720‐32. [MEDLINE: ] - PubMed
Heran 2008
Higgins 2002
-
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [MEDLINE: ] - PubMed
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org, 2011.
Kellick 1997
-
- Kellick KA, Burns K, Mcandrew E, Haberl E, Hook N, Ellis AK. Focus on atorvastatin: An HMG‐CoA reductase inhibitor for lowering both elevated LDL cholesterol and triglycerides in hypercholesterolemic patients. Formulary 1997;32(4):352‐63. [EMBASE: 27183679]
Kreatsoulas 2010
Law 2003
Liao 2005
Mills 2008
-
- Mills EJ, Rachlis B, Wu P, Devereaux PJ, Arora P, Perri D. Primary prevention of cardiovascular mortality and events with statin treatments: a network meta‐analysis involving more than 65,000 patients. Journal of the American College of Cardiology 2008;52(22):1769‐81. [MEDLINE: ] - PubMed
Moghadasian 1999
-
- Moghadasian MH. Clinical pharmacology of 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase inhibitors. Life Sciences 1999;65(13):1329‐37. [MEDLINE: ] - PubMed
Roger 2011
-
- Roger VL, Go AS, Lloyd‐Jones DM, Adams RJ, Berry JD, Brown TM, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics ‐‐ 2011 update: a report from the American Heart Association. Circulation 2011;123(4):e18‐e209. [MEDLINE: ] - PMC - PubMed
Schaefer 2004
-
- Schaefer EJ, McNamara JR, Tayler T, Daly JA, Gleason JL, Seman LJ, Ferrari A, Rubenstein JJ. Comparisons of effects of statins (atorvastatin, fluvastatin, lovastatin, pravastatin, and simvastatin) on fasting and postprandial lipoproteins in patients with coronary heart disease versus control subjects. American Journal of Cardiology 2004;93(1):31‐9. [MEDLINE: ] - PubMed
Schectman 1996
-
- Schectman G, Hiatt J. Dose‐response characteristics of cholesterol‐lowering drug therapies: implications for treatment. Annals of Internal Medicine 1996;125(12):990‐1000. [MEDLINE: ] - PubMed
Smith 2009
-
- Smith MEB, Lee NJ, Haney E, Carson S. Drug Class Review HMG‐CoA Reductase Inhibitors (Statins) and Fixed‐dose Combination Products Containing a Statin. Final Report Update 5, 2009. www.ncbi.nlm.nih.gov/books/NBK47273/pdf/TOC.pdf (last accessed 8 November 2012). - PubMed
Sterne 2011
-
- Sterne JAC, Egger M, Moher D on behalf of the Cochrane Bias Methods Group (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org, 2011.
Taylor 2013
Thompson 2005
-
- Thompson JF, Man M, Johnson KJ, Wood LS, Lira ME, Lloyd DB, Banerjee P, Milos PM, Myrand SP, Paulauskis J, Milad MA, Sasiela WJ. An association study of 43 SNPs in 16 candidate genes with atorvastatin response. Pharmacogenomics Journal 2005;5(6):352‐8. [MEDLINE: ] - PubMed
Tsang 2002
-
- Tsang MB, Adams SP, Jauca C, Wright JM. In Some Systematic Reviews Placebos May Not Be Necessary: An Example From a Statin Dose‐Response Study. 10th Cochrane Colloquium Abstracts; 2002 31 Jul‐3 Aug; Stavanger, Norway. 2002:Poster 29.
Ward 2007
-
- Ward S, Lloyd Jones M, Pandor A, Holmes M, Ara R, Ryan A, Yeo W, Payne N. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technology Assessment 2007;11(14):1‐160, iii‐iv. [MEDLINE: ] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

