Protein interference applications in cellular and developmental biology using DARPins that recognize GFP and mCherry
- PMID: 25416061
- PMCID: PMC4265764
- DOI: 10.1242/bio.201410041
Protein interference applications in cellular and developmental biology using DARPins that recognize GFP and mCherry
Abstract
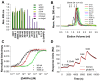
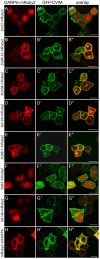
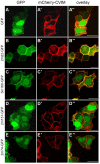
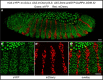
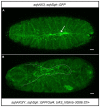
Protein-protein interactions are crucial for cellular homeostasis and play important roles in the dynamic execution of biological processes. While antibodies represent a well-established tool to study protein interactions of extracellular domains and secreted proteins, as well as in fixed and permeabilized cells, they usually cannot be functionally expressed in the cytoplasm of living cells. Non-immunoglobulin protein-binding scaffolds have been identified that also function intracellularly and are now being engineered for synthetic biology applications. Here we used the Designed Ankyrin Repeat Protein (DARPin) scaffold to generate binders to fluorescent proteins and used them to modify biological systems directly at the protein level. DARPins binding to GFP or mCherry were selected by ribosome display. For GFP, binders with KD as low as 160 pM were obtained, while for mCherry the best affinity was 6 nM. We then verified in cell culture their specific binding in a complex cellular environment and found an affinity cut-off in the mid-nanomolar region, above which binding is no longer detectable in the cell. Next, their binding properties were employed to change the localization of the respective fluorescent proteins within cells. Finally, we performed experiments in Drosophila melanogaster and Danio rerio and utilized these DARPins to either degrade or delocalize fluorescently tagged fusion proteins in developing organisms, and to phenocopy loss-of-function mutations. Specific protein binders can thus be selected in vitro and used to reprogram developmental systems in vivo directly at the protein level, thereby bypassing some limitations of approaches that function at the DNA or the RNA level.
Keywords: DARPin; GFP; Protein interference; mCherry.
© 2014. Published by The Company of Biologists Ltd.
Conflict of interest statement
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

