The role of inflammation in hypoxic pulmonary hypertension: from cellular mechanisms to clinical phenotypes
- PMID: 25416383
- PMCID: PMC4338929
- DOI: 10.1152/ajplung.00238.2014
The role of inflammation in hypoxic pulmonary hypertension: from cellular mechanisms to clinical phenotypes
Abstract
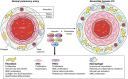
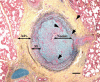
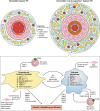
Hypoxic pulmonary hypertension (PH) comprises a heterogeneous group of diseases sharing the common feature of chronic hypoxia-induced pulmonary vascular remodeling. The disease is usually characterized by mild to moderate pulmonary vascular remodeling that is largely thought to be reversible compared with the progressive irreversible disease seen in World Health Organization (WHO) group I disease. However, in these patients, the presence of PH significantly worsens morbidity and mortality. In addition, a small subset of patients with hypoxic PH develop "out-of-proportion" severe pulmonary hypertension characterized by pulmonary vascular remodeling that is irreversible and similar to that in WHO group I disease. In all cases of hypoxia-related vascular remodeling and PH, inflammation, particularly persistent inflammation, is thought to play a role. This review focuses on the effects of hypoxia on pulmonary vascular cells and the signaling pathways involved in the initiation and perpetuation of vascular inflammation, especially as they relate to vascular remodeling and transition to chronic irreversible PH. We hypothesize that the combination of hypoxia and local tissue factors/cytokines ("second hit") antagonizes tissue homeostatic cellular interactions between mesenchymal cells (fibroblasts and/or smooth muscle cells) and macrophages and arrests these cells in an epigenetically locked and permanently activated proremodeling and proinflammatory phenotype. This aberrant cellular cross-talk between mesenchymal cells and macrophages promotes transition to chronic nonresolving inflammation and vascular remodeling, perpetuating PH. A better understanding of these signaling pathways may lead to the development of specific therapeutic targets, as none are currently available for WHO group III disease.
Keywords: chronic nonresolving inflammation; fibroblasts; hypoxia; hypoxic pulmonary hypertension; inflammation; macrophages.
Copyright © 2015 the American Physiological Society.
Figures
References
-
- Aaronson PI, Robertson TP, Ward JP. Endothelium-derived mediators and hypoxic pulmonary vasoconstriction. Respir Physiol Neurobiol 132: 107–120, 2002. - PubMed
-
- Abe K, Toba M, Alzoubi A, Ito M, Fagan KA, Cool CD, Voelkel NF, McMurtry IF, Oka M. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation 121: 2747–2754, 2010. - PubMed
-
- Adkins WK, Taylor AE. Role of xanthine oxidase and neutrophils in ischemia-reperfusion injury in rabbit lung. J Appl Physiol 69: 2012–2018, 1990. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

