Selective reporting bias of harm outcomes within studies: findings from a cohort of systematic reviews
- PMID: 25416499
- PMCID: PMC4240443
- DOI: 10.1136/bmj.g6501
Selective reporting bias of harm outcomes within studies: findings from a cohort of systematic reviews
Abstract
Objective: To determine the extent and nature of selective non-reporting of harm outcomes in clinical studies that were eligible for inclusion in a cohort of systematic reviews.
Design: Cohort study of systematic reviews from two databases.
Setting: Outcome reporting bias in trials for harm outcomes (ORBIT II) in systematic reviews from the Cochrane Library and a separate cohort of systematic reviews of adverse events.
Participants: 92 systematic reviews of randomised controlled trials and non-randomised studies published in the Cochrane Library between issue 9, 2012 and issue 2, 2013 (Cochrane cohort) and 230 systematic reviews published between 1 January 2007 and 31 December 2011 in other publications, synthesising data on harm outcomes (adverse event cohort).
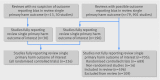
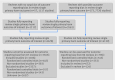
Methods: A 13 point classification system for missing outcome data on harm was developed and applied to the studies.
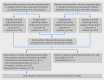
Results: 86% (79/92) of reviews in the Cochrane cohort did not include full data from the main harm outcome of interest of each review for all of the eligible studies included within that review; 76% (173/230) for the adverse event cohort. Overall, the single primary harm outcome was inadequately reported in 76% (705/931) of the studies included in the 92 reviews from the Cochrane cohort and not reported in 47% (4159/8837) of the 230 reviews in the adverse event cohort. In a sample of primary studies not reporting on the single primary harm outcome in the review, scrutiny of the study publication revealed that outcome reporting bias was suspected in nearly two thirds (63%, 248/393).
Conclusions: The number of reviews suspected of outcome reporting bias as a result of missing or partially reported harm related outcomes from at least one eligible study is high. The declaration of important harms and the quality of the reporting of harm outcomes must be improved in both primary studies and systematic reviews.
© Saini et al 2014.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures
Comment in
-
Reporting of harms in systematic reviews and their primary studies.BMJ. 2014 Nov 21;349:g6819. doi: 10.1136/bmj.g6819. BMJ. 2014. PMID: 25416500 No abstract available.
References
-
- Hutton JL, Williamson PR. Bias in meta-analysis due to outcome variable selection within studies. Appl Stat 2000;49:359-70.
-
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. - PubMed
-
- Jonsson U, Alaie I, Parling T, Arnberg FK. Reporting of harms in randomized controlled trials of psychological interventions for mental and behavioural disorders: a review of current practice. Contemp Clin Trials 2014;38:1-8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical