Vancomycin population pharmacokinetics during extracorporeal membrane oxygenation therapy: a matched cohort study
- PMID: 25416535
- PMCID: PMC4256896
- DOI: 10.1186/s13054-014-0632-8
Vancomycin population pharmacokinetics during extracorporeal membrane oxygenation therapy: a matched cohort study
Abstract
Introduction: The aim of this study was to describe the population pharmacokinetics of vancomycin in critically ill patients treated with and without extracorporeal membrane oxygenation (ECMO).
Methods: We retrospectively reviewed data from critically ill patients treated with ECMO and matched controls who received a continuous infusion of vancomycin (35 mg/kg loading dose over 4 hours followed by a daily infusion adapted to creatinine clearance, CrCl)). The pharmacokinetics of vancomycin were described using non-linear mixed effects modeling.
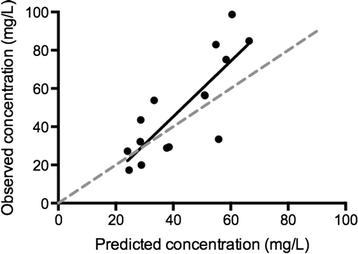
Results: We compared 11 patients treated with ECMO with 11 well-matched controls. Drug dosing was similar between groups. The median interquartile range (IQR) vancomycin concentrations in ECMO and non-ECMO patients were 51 (28 to 71) versus 45 (37 to 71) mg/L at 4 hours; 23 (16 to 38) versus 29 (21 to 35) mg/L at 12 hours; 20 (12 to 36) versus 23 (17-28) mg/L at 24 hours (ANOVA, P = 0.53). Median (ranges) volume of distribution (Vd) was 99.3 (49.1 to 212.3) and 92.3 (22.4 to 149.4) L in ECMO and non-ECMO patients, respectively, and clearance 2.4 (1.7 to 4.9) versus 2.3 (1.8 to 3.6) L/h (not significant). Insufficient drug concentrations (that is drug levels < 20 mg/dL) were more common in the ECMO group. The pharmacokinetic model (non-linear mixed effects modeling) was prospectively validated in five additional ECMO-treated patients over a 6-month period. Linear regression analysis comparing the observed concentrations and those predicted using the model showed good correlation (r(2) of 0.67; P < 0.001).
Conclusions: Vancomycin concentrations were similar between ECMO and non-ECMO patients in the early phase of therapy. ECMO treatment was not associated with significant changes in Vd and drug clearance compared with the control patients.
Figures



References
-
- Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, Firmin RK, Elbourne D, CESAR trial collaboration Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. - DOI - PubMed
-
- Shekar K, Roberts JA, Welch S, Buscher H, Rudham S, Burrows F, Ghassabian S, Wallis SC, Levkovich B, Pellegrino V, McGuinness S, Parke R, Gilder E, Barnett AG, Walsham J, Mullany DV, Fung YL, Smith MT, Fraser JF. Antibiotic, Sedative and Analgesic Pharmacokinetics during Extracorporeal Membrane Oxygenation: a multi-centre study to optimise drug therapy during ECMO. BMC Anesthesiol. 2012;12:29. doi: 10.1186/1471-2253-12-29. - DOI - PMC - PubMed
-
- Roberts DM, Roberts JA, Roberts MS, Liu X, Nair P, Cole L, Lipman J, Bellomo R, RENAL Replacement Therapy Study Investigators Variability of antibiotic concentrations in critically ill patients receiving continuous renal replacement therapy – a multicentre pharmacokinetic study. Crit Care Med. 2012;40:1523–1528. doi: 10.1097/CCM.0b013e318241e553. - DOI - PubMed
-
- Udy AA, Varghese JM, Altukroni M, Briscoe S, McWhinney BC, Ungerer JP, Lipman J, Roberts JA. Sub-therapeutic initial beta-lactam concentrations in select critically ill patients: association between augmented renal clearance and low trough drug concentrations. Chest. 2012;142:30–39. doi: 10.1378/chest.11-1671. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

