Differential function of Gγ13 in rod bipolar and ON cone bipolar cells
- PMID: 25416620
- PMCID: PMC4386959
- DOI: 10.1113/jphysiol.2014.281196
Differential function of Gγ13 in rod bipolar and ON cone bipolar cells
Abstract
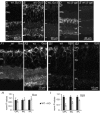
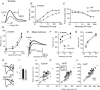
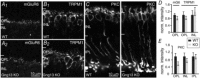
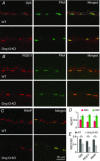
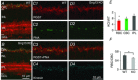
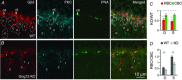
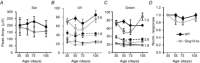
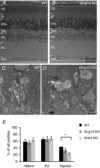
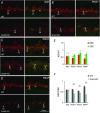
Heterotrimeric G-proteins (comprising Gα and Gβγ subunits) are critical for coupling of metabotropic receptors to their downstream effectors. In the retina, glutamate released from photoreceptors in the dark activates metabotropic glutamate receptor 6 (mGluR6) receptors in ON bipolar cells; this leads to activation of Go , closure of transient receptor potential melastatin 1 channels and hyperpolarization of these cells. Go comprises Gαo , Gβ3 and a Gγ. The best Gγ candidate is Gγ13, although functional data to support this are lacking. Thus, we tested Gγ13 function by generating Gng13(-/-) knockout (KO) mice, recording electroretinograms (ERG) and performing immunocytochemical staining. The amplitude of scotopic ERG b-waves in KO mice was lower than in wild-type (WT) mice. Furthermore, in both KO and WT mice, the ERG b-wave decreased with age; this decrease was much more pronounced in KO mice. By contrast, the photopic ERG b-waves in KO mice were hardly affected at any age. In KO mice retinas, immunostaining for Gβ3 and for the GTPase activating proteins RGS7, RGS11, R9AP and Gβ5 decreased significantly in rod bipolar cells but not in ON cone bipolar cells. Staining for Gαo and certain other cascade elements decreased only slightly. Analysis of our ON bipolar cDNA library showed that these cells express mRNAs for Gγ5, Gγ10 and Gγ11. Quantitative RT-PCR of retinal cDNA showed greater values for these transcripts in retinas of KO mice, although the difference was not significant. Our results suggest that Gγ13 contributes to mGluR6 signalling in rod bipolar cells more than in ON cone bipolar cells, and that this contribution includes both coupling the receptor and maintaining a stable localization of the mGluR6-related cascade elements.
© 2014 The Authors. The Journal of Physiology © 2014 The Physiological Society.
Figures



Comment in
-
All ON pathways are not alike.J Physiol. 2015 Apr 1;593(7):1527-8. doi: 10.1113/JP270006. J Physiol. 2015. PMID: 25828643 Free PMC article. No abstract available.
References
-
- Audo I, Kohl S, Leroy BP, Munier FL, Guillonneau X, Mohand-Said S, Bujakowska K, Nandrot EF, Lorenz B, Preising M, Kellner U, Renner AB, Bernd A, Antonio A, Moskova-Doumanova V, Lancelot ME, Poloschek CM, Drumare I, Defoort-Dhellemmes S, Wissinger B, Leveillard T, Hamel CP, Schorderet DF, De Baere E, Berger W, Jacobson SG, Zrenner E, Sahel JA, Bhattacharya SS. Zeitz C. TRPM1 is mutated in patients with autosomal-recessive complete congenital stationary night blindness. Am J Human Gen. 2009;85:720–729. - PMC - PubMed
-
- Blake BL, Wing MR, Zhou JY, Lei Q, Hillmann JR, Behe CI, Morris RA, Harden TK, Bayliss DA, Miller RJ. Siderovski DP. G beta association and effector interaction selectivities of the divergent G gamma subunit G gamma(13) J Biolog Chem. 2001;276:49267–49274. - PubMed
-
- Boyer JL, Waldo GL. Harden TK. Beta gamma-subunit activation of G-protein-regulated phospholipase C. J Biol Chem. 1992;267:25451–25456. - PubMed
-
- Breton ME, Schueller AW, Lamb TD. Pugh EN., Jr Analysis of ERG a-wave amplification and kinetics in terms of the G-protein cascade of phototransduction. Invest Ophthalmol Vis Sci. 1994;35:295–309. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

