Androgen Modulation of Hippocampal Structure and Function
- PMID: 25416742
- PMCID: PMC5002217
- DOI: 10.1177/1073858414558065
Androgen Modulation of Hippocampal Structure and Function
Abstract
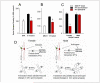
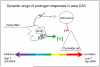
Androgens have profound effects on hippocampal structure and function, including induction of spines and spine synapses on the dendrites of CA1 pyramidal neurons, as well as alterations in long-term synaptic plasticity (LTP) and hippocampally dependent cognitive behaviors. How these effects occur remains largely unknown. Emerging evidence, however, suggests that one of the key elements in the response mechanism may be modulation of brain-derived neurotrophic factor (BDNF) in the mossy fiber (MF) system. In male rats, orchidectomy increases synaptic transmission and excitability in the MF pathway. Testosterone reverses these effects, suggesting that testosterone exerts tonic suppression on MF BDNF levels. These findings suggest that changes in hippocampal function resulting from declining androgen levels may reflect the outcome of responses mediated through normally balanced, but opposing, mechanisms: loss of androgen effects on the hippocampal circuitry may be compensated, at least in part, by an increase in BDNF-dependent MF plasticity.
Keywords: BDNF; CA3; hippocampus; mossy fibers; testosterone.
© The Author(s) 2014.
Figures



References
-
- Ahmadiani A, Mandgary A, Sayyah M. Anticonvulsant effect of flutamide on seizures induced by pentylenetetrazole: involvement of benzodiazepine receptors. Epilepsia. 2003;44:629–35. - PubMed
-
- Alibhai SM, Breunis H, Timilshina N, Marzouk S, Stewart D, Tannock I. Impact of androgen-deprivation therapy on cognitive function in men with nonmetastatic prostate cancer. J Clin Oncol. 2010;28:5030–7. others. - PubMed
-
- Alsemari A. Hypogonadism and neurological diseases. Neurol Sci. 2013;34:629–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

