Belatacept for kidney transplant recipients
- PMID: 25416857
- PMCID: PMC6486198
- DOI: 10.1002/14651858.CD010699.pub2
Belatacept for kidney transplant recipients
Abstract
Background: Most people who receive a kidney transplant die from either cardiovascular disease or cancer before their transplant fails. The most common reason for someone with a kidney transplant to lose the function of their transplanted kidney necessitating return to dialysis is chronic kidney transplant scarring. Immunosuppressant drugs have side effects that increase risks of cardiovascular disease, cancer and chronic kidney transplant scarring. Belatacept may provide sufficient immunosuppression while avoiding unwanted side effects of other immunosuppressant drugs. However, high rates of post-transplant lymphoproliferative disease (PTLD) have been reported when belatacept is used in particular kidney transplant recipients at high dosage.
Objectives: 1) Compare the relative efficacy of belatacept versus any other primary immunosuppression regimen for preventing acute rejection, maintaining kidney transplant function, and preventing death. 2) Compare the incidence of several adverse events: PTLD; other malignancies; chronic transplant kidney scarring (IF/TA); infections; change in blood pressure, lipid and blood sugar control. 3) Assess any variation in effects by study, intervention and recipient characteristics, including: differences in pre-transplant Epstein Barr virus serostatus; belatacept dosage; and donor-category (living, standard criteria deceased, or extended criteria deceased).
Search methods: We searched the Cochrane Renal Group's Specialised Register to 1 September 2014 through contact with the Trials' Search Co-ordinator using search terms relevant to this review.
Selection criteria: Randomised controlled trials (RCT) that compared belatacept versus any other immunosuppression regimen in kidney transplant recipients were eligible for inclusion.
Data collection and analysis: Two authors independently extracted data for study quality and transplant outcomes and synthesized results using random effects meta-analysis, expressed as risk ratios (RR) and mean differences (MD), both with 95% confidence intervals (CI). Subgroup analyses and univariate meta-regression were used to investigate potential heterogeneity.
Main results: We included five studies that compared belatacept and calcineurin inhibitors (CNI) that reported data from a total of 1535 kidney transplant recipients. Of the five studies, three (478 participants) compared belatacept and cyclosporin and two (43 recipients) compared belatacept and tacrolimus. Co-interventions included basiliximab (4 studies, 1434 recipients); anti-thymocyte globulin (1 study, 89 recipients); alemtuzumab (1 study, 12 recipients); mycophenolate mofetil (MMF, 5 studies, 1509 recipients); sirolimus (1 study, 26 recipients) and prednisone (5 studies, 1535 recipients).Up to three years following transplant, belatacept and CNI-treated recipients were at similar risk of dying (4 studies, 1516 recipients: RR 0.75, 95% CI 0.39 to 1.44), losing their kidney transplant and returning to dialysis (4 studies, 1516 recipients: RR 0.91, 95% CI 0.61 to 1.38), and having an episode of acute rejection (4 studies, 1516 recipients: RR 1.56, 95% CI 0.85 to 2.86). Belatacept-treated kidney transplant recipients were 28% less likely to have chronic kidney scarring (3 studies, 1360 recipients: RR 0.72, 95% CI 0.55 to 0.94) and also had better graft function (measured glomerular filtration rate (GFR) (3 studies 1083 recipients): 10.89 mL/min/1.73 m², 95% CI 4.01 to 17.77; estimated GFR (4 studies, 1083 recipients): MD 9.96 mL/min/1.73 m², 95% CI 3.28 to 16.64) than CNI-treated recipients. Blood pressure was lower (systolic (2 studies, 658 recipients): MD -7.51 mm Hg, 95% CI -10.57 to -4.46; diastolic (2 studies, 658 recipients): MD -3.07 mm Hg, 95% CI -4.83 to -1.31, lipid profile was better (non-HDL (3 studies 1101 recipients): MD -12.25 mg/dL, 95% CI -17.93 to -6.57; triglycerides (3 studies 1101 recipients): MD -24.09 mg/dL, 95% CI -44.55 to -3.64), and incidence of new-onset diabetes after transplant was reduced by 39% (4 studies (1049 recipients): RR 0.61, 95% CI 0.40 to 0.93) among belatacept-treated versus CNI-treated recipients.Risk of PTLD was similar in belatacept and CNI-treated recipients (4 studies, 1516 recipients: RR 2.79, 95% CI 0.61 to 12.66) and was no different among recipients who received different belatacept dosages (high versus low dosage: ratio of risk ratios (RRR) 1.06, 95% CI 0.11 to 9.80, test of difference = 0.96) or among those who were Epstein Barr virus seronegative compared with those who were seropositive before their kidney transplant (seronegative versus seropositive; RRR 1.49, 95% CI 0.15 to 14.76, test for difference = 0.73).The belatacept dose used (high versus low), type of donor kidney the recipient received (extended versus standard criteria) and whether the kidney transplant recipient received tacrolimus or cyclosporin made no difference to kidney transplant survival, incidence of acute rejection or estimated GFR. Selective outcome reporting meant that data for some key subgroup comparisons were sparse and that estimates of the effect of treatment in these groups of recipients remain imprecise.
Authors' conclusions: There is no evidence of any difference in the effectiveness of belatacept and CNI in preventing acute rejection, graft loss and death, but treatment with belatacept is associated with less chronic kidney scarring and better kidney transplant function. Treatment with belatacept is also associated with better blood pressure and lipid profile and a lower incidence of diabetes versus treatment with a CNI. Important side effects (particularly PTLD) remain poorly reported and so the relative benefits and harms of using belatacept remain unclear. Whether short-term advantages of treatment with belatacept are maintained over the medium- to long-term or translate into better cardiovascular outcomes or longer kidney transplant survival with function remains unclear. Longer-term, fully reported and published studies comparing belatacept versus tacrolimus are needed to help clinicians decide which patients might benefit most from using belatacept.
Conflict of interest statement
Philip Masson: none known
Lorna Henderson: none known
Jeremy R Chapman: none known
Jonathan C Craig: none known
Angela C Webster: none known
Figures

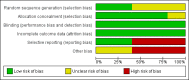
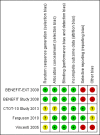




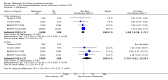









Update of
References
References to studies included in this review
BENEFIT‐EXT 2009 {published data only}
-
- Charpentier B, Grinyo J, Medina‐Pestana J, Vanrenterghem I, Vincente F, Dong Y, et al. 3‐year safety profile of belatacept in kidney transplant recipients from the BENEFIT and BENEFIT‐EXT studies [abstract no: O‐242]. Transplant International 2011;24(Suppl 2):68‐9. [EMBASE: 70527318]
-
- Charpentier B, Medina Pestana JO, Del CR, Rostaing L, Grinyo J, Vanrenterghem Y, et al. Long‐term exposure to belatacept in recipients of extended criteria donor kidneys. American Journal of Transplantation 2013;13(11):2884‐91. [MEDLINE: ] - PubMed
-
- Charpentier B, Vincenti F, Rice K, Campistol J, Duan T, Pupim L, et al. Three‐year outcomes in patients with delayed graft function in phase III studies of belatacept vs cyclosporine in kidney transplantation (BENEFIT and BENEFIT‐EXT) [abstract no: PO27.076]. 24th International Congress of the Transplantation Society (TTS); 2012 Jul 15‐19; Berlin, Germany. 2012. [EMBASE: 71251772]
-
- Dobbels F, Wong S, Joo S, Kalsekar A. Health‐related quality of life after kidney transplantation: results from belatacept clinical trials [abstract no: 1099]. American Journal of Transplantation 2011;11(Suppl 2):352‐3. [EMBASE: 70406145]
BENEFIT Study 2008 {published data only}
-
- Blancho G, Budde K, Merville P, Moal M, Rostaing L, Harler M, et al. Outcomes at 3 years in EBV+ European subpopulations from BENEFIT and BENEFIT‐EXT [abstract no: A198]. American Journal of Transplantation 2014;14(Suppl 5):455. [EMBASE: 71545053]
-
- Bray R, Gebel H, Brannon P, Ganguly B, Townsend R, Meier‐Kriesche U, et al. Evaluation of donor‐specific antibodies through 5 years with belatacept in BENEFIT and BENEFIT‐EXT [abstract no: 2163]. American Journal of Transplantation 2014;14(Suppl 3):117. [EMBASE: 71543898]
-
- Bresnahan B, Vincenti F, Grinyo J, Charpentier B, Russo GD, Garg P, et al. Renal benefit of belatacept versus cyclosporine in kidney transplant patients is not impacted by acute rejection (BENEFIT study) [abstract no: 56]. American Journal of Transplantation 2010;10(Suppl 2):14. [EMBASE: 70081079]
-
- Charpentier B, Grinyo J, Medina‐Pestana J, Vanrenterghem I, Vincente F, Dong Y, et al. 3‐year safety profile of belatacept in kidney transplant recipients from the BENEFIT and BENEFIT‐EXT studies [abstract no: O‐242]. Transplant International 2011;24(Suppl 2):68‐9. [EMBASE: 70527318]
CTOT‐10 Study 2013 {published data only}
-
- Newell K, Mannon R, Mehta A, Tomlanovich S, Morrison Y, Ikle D, et al. Long‐term calcineurin inhibitor (CNI) and corticosteroid (CS) avoidance using belatacept: the CTOT‐10 experience [abstract no: 15]. American Journal of Transplantation 2013;13(Suppl 5):34. [EMBASE: 71056592]
-
- Suessmuth Y, Stempora L, Johnson B, Cheeseman J, Morrison Y, Bridges N, et al. Comparison of baseline and day 28 post transplant renal allograft samples from the CTOT‐10 belatacept study [abstract no: A630]. American Journal of Transplantation 2013;13(Suppl S5):225. [EMBASE: 71057206]
Ferguson 2010 {published data only}
-
- Ferguson R, Grinyo J, Vincenti F, Kaufman DB, Woodle ES, Marder BA, et al. Immunosuppression with belatacept‐based, corticosteroid‐avoiding regimens in de novo kidney transplant recipients. American Journal of Transplantation 2011;11(1):66‐76. [MEDLINE: ] - PubMed
-
- Ferguson R, Vincenti F, Kaufman D, Woodle ES, Marder BA, Citterio F, et al. Immunosuppression with belatacept‐based, CNI‐avoiding and steroid‐avoiding regimens vs a tacrolimus‐based, steroid‐avoiding regimen in kidney transplant patients: results of a 1‐year, randomized study [abstract no: 1436]. Transplantation 2010;90(Suppl):156. [EMBASE: 71531403]
-
- Ferguson R, Vincenti F, Kaufman DB, Woodle ES, Marder BA, Citterio F, et al. Immunosuppression with belatacept‐based, CNI‐avoiding and steroid‐avoiding regimens vs a tacrolimus‐based, steroid‐avoiding regimen in kidney transplant patients: results of a 1‐year, randomized study [abstract no: 372]. American Journal of Transplantation 2010;10(Suppl 4):150. [EMBASE: 70463733]
-
- Grinyo J, Marder B, Harler M, Vincenti F, Citterio F, Nori U, et al. Outcomes at 4 years in a randomized trial evaluating belatacept‐based regimens with simultaneous CNI and steroid‐avoidance in kidney transplant [abstract no: 2162]. American Journal of Transplantation 2014;14(Suppl 3):116. [EMBASE: 71543897]
-
- Grinyo J, Marder B, Harler M, Vincenti F, Citterio F, Nori U, et al. Outcomes at 4 years in a randomized trial evaluating belatacept‐based regimens with simultaneous CNI and steroid‐avoidance in kidney transplant [abstract]. Transplantation 2014;98(Suppl 1):116‐7. [EMBASE: 71543897]
Vincenti 2005 {published data only}
-
- Blancho G, Nashan B, Vincenti F, Charpentier B. Selective co‐stimulation blockade with belatacept (LEA29Y) shows improved preservation of renal function at 12 months vs cyclosporine [abstract]. Transplant International 2005;18(Suppl 1):22.
-
- Charpentier B, Grannas G, Pearson T, Soulillou J, Vanrenterghem Y, Friend P, et al. Co‐stimulation blockade with LEA29Y in renal transplant: improved renal function and cv/metabolic profile at 6 months compared with cyclosporine [abstract]. Transplantation 2004;78(2 Suppl):84.
-
- Charpentier B, Larsen C, Grinyo J, Muehlbacher F, Blancho G, Grannas G, et al. Final results from the long term extension (LTE) of the belatacept phase 2 study in kidney transplantation [abstract no: 2164]. American Journal of Transplantation 2014;14(Suppl 3):117. [EMBASE: 71543899]
-
- Charpentier B, Larsen C, Grinyo J, Muehlbacher F, Blancho G, Grannas G, et al. Final results from the long‐term extension (LTE) of the belatacept phase 2 study in kidney transplantation [abstract]. Transplantation 2014;98(Suppl 1):117. [EMBASE: 71543899]
References to studies excluded from this review
Kamar 2013 {published data only}
-
- Kamar N, Rial M, Alberu J, Steinberg S, Manfro R, Nainan G, et al. Three‐years outcomes after switching to belatacept from calcineurin inhibitor in stable kidney transplant recipients [abstract]. Transplant International 2013;26(Suppl 3):22. [EMBASE: 71356163]
-
- Kamar N, Rial M, Alberu J, Steinberg SM, Manfro R, Nainan G, et al. 3‐year outcomes after switching to belatacept from a calcineurin inhibitor in stable kidney transplant recipients [abstract]. Transplant International 2013;26(Suppl 2):44. [EMBASE: 71359102]
Kirk 2012 {published data only}
-
- Kirk A, Meas S, Xu H, Mehta A, Guasch A, Cheeseman J, et al. Kidney transplantation using alemtuzumab induction and belatacept/sirolimus maintenance therapy [abstract no: 56]. American Journal of Transplantation 2011;11(Suppl 2):45. [EMBASE: 70405091]
-
- Kirk A, Mehta A, Guasch A, Mead S, Xu H, Cheeseman J, et al. Kidney transplantation using alemtuzumab induction and belatacept/sirolimus maintenance therapy [abstract no: 560]. American Journal of Transplantation 2012;12(Suppl 3):197. [EMBASE: 70746514]
Rostaing 2011 {published data only}
-
- Grinyo J, Alberu J, Contieri FL, Manfro RC, Mondragon G, Nainan G, et al. Improvement in renal function in kidney transplant recipients switched from cyclosporine or tacrolimus to belatacept: 2‐year results from the long‐term extension of a phase II study. Transplant International 2012;25(10):1059‐64. [MEDLINE: ] - PubMed
-
- Grinyo J, Nainan G, Carmen RM, Steinberg S, Vincenti F, Dong Y, et al. Renal function at 2 years in kidney transplant recipients switched from ciclosporin or tacrolimus to belatacept: results from the long‐term extension of a phase II study [abstract]. British Transplantation Society (BTS). 15th Annual Congress; 2012 Feb 22‐24; Glasgow, UK. 2012.
-
- Grinyo J, Nainan G, Carmen RM, Steinberg S, Vincenti F, Dong Y, et al. Renal function at 2 years in kidney transplant recipients switched from cyclosporine or tacrolimus to belatacept: results from the long term extension of a phase II study [abstract no: O‐245]. Transplant International 2011;24(Suppl 2):70. - PubMed
-
- Grinyo J, Nainan G, Carmen Rial M, Steinberg S, Vincenti F, Dong Y, et al. Renal function at 2 years in kidney transplant recipients switched from cyclosporine or tacrolimus to belatacept: results from the long‐term extension of a phase II study [abstract no: 567]. NDT Plus 2011;4(Suppl 2):4.s2.43. - PubMed
-
- Grinyo J, Nainan G, Rial M, Steinberg S, Vincenti F, Dong Y, et al. Renal function at 2 years in kidney transplant recipients switched from cyclosporine or tacrolimus to belatacept: Results: from the long‐term extension of a phase II study [abstract no: 226]. American Journal of Transplantation 2011;11(Suppl 2):99. [EMBASE: 70405261] - PubMed
References to ongoing studies
EudraCT2006‐00311417 {published data only}
-
- EUCTR2006‐003114‐17. A randomized, open‐label, multicenter, parallel‐group study of belatacept‐based corticosteroid‐free regimens in renal transplant. www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number (accessed 4 June 2014).
EudraCT2011‐00616240 {published data only}
-
- Moreso F. New‐onset diabetes mellitus after renal transplantation. a multicentre, prospective, randomized, open study to evaluate belatacept‐based versus tacrolimus‐based immunosuppression. www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number (accessed 4 June 2014).
EudraCT2013‐00117820 {published data only}
-
- Karlberg E. Cardiovascular (CV) risk prediction and CV biomarkers in renal transplant recipients treated with belatacept compared to calcineurin inhibitors (CNI). Open randomized 12 month study. www.clinicaltrialsregister.eu/ctr‐search/search?query=2013‐001178‐20 (accessed 4 June 2014).
NCT01729494 {published data only}
-
- Woodle S. Randomized, open label, multicenter study of belatacept‐based early steroid withdrawal regimen with alemtuzumab or RATG induction compared to tacrolimus‐based early steroid withdrawal regimen with RATG induction in renal transplantation [Belatacept early steroid withdrawal trial]. www.clinicaltrials.gov/ct2/show/NCT01729494 (accessed 26 May 2014).
NTR4242 {published data only}
-
- Hesselink DA. Immune monitoring to characterize t‐cell responses of kidney transplant patients during costimulation blockade by belatacept. www.trialregister.nl/trialreg/admin/rctview asp?TC=4242 (accessed 4 June 2014).
Additional references
ANZDATA 2012
-
- Clayton P, Hurst K, McDonald S, Chadban S. ANZDATA Registry Report 2012. Chapter 8: Transplantation. www.anzdata.org.au/anzdata/AnzdataReport/35thReport/2012c08_transplants_... (accessed 11 November 2014):2‐27.
Begg 1996
-
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. JAMA 1996;276(8):637‐9. [MEDLINE: ] - PubMed
Buell 2005
-
- Buell JF, Gross TG, Woodle ES. Malignancy after transplantation. Transplantation 2005;80(2 Suppl):S254‐64. [MEDLINE: ] - PubMed
Dobbels 2014
-
- Dobbels F, Wong S, Min Y, Sam J, Kalsekar A. Beneficial effect of belatacept on health‐related quality of life and perceived side effects: results from the BENEFIT and BENEFIT‐EXT trials. Transplantation 2014;98(9):960‐8. [MEDLINE: ] - PubMed
Ekberg 2009
-
- Ekberg H, Bernasconi C, Tedesco‐Silva H, Vitko S, Hugo C, Demirbas A, et al. Calcineurin inhibitor minimization in the Symphony study: observational results 3 years after transplantation. American Journal of Transplantation 2009;9(8):1876‐85. [MEDLINE: ] - PubMed
Flechner 2011
-
- Felchner SM, Glyda M, Cockfield S, Grinyo J, Legendre C, Russ G, et al. The ORION study: comparison of two sirolimus‐based regimens versus tacrolimus and mycophenolate mofetil in renal allograft recipients. American Journal of Transplantation 2011;11(8):1633‐44. [MEDLINE: ] - PubMed
Gloor 2009
-
- Gloor JM, Winters JL, Cornell LD, Fix LA, DeGoey SR, Knauer RM, et al. Baseline donor‐specific antibody levels and outcomes in positive crossmatch kidney transplantation. American Journal of Transplantation 2010;10(3):582‐9. [MEDLINE: ] - PubMed
Godlee 2012
-
- Godlee F, Groves T. The new BMJ policy on sharing data from drug and device trials. BMJ 2012;345:e37888. - PubMed
Hidalgo 2009
-
- Hidalgo LG, Campbell PM, Sis B, Einecke G, Mengel M, Chang J, et al. De novo donor‐specific antibody at the time of kidney transplant biopsy associates with microvascular pathology and late graft failure. American Journal of Transplantation 2009;9(11):2532‐41. [MEDLINE: ] - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Masson 2013
-
- Masson P, Duthie FA, Ruster LP, Kelly PJ, Merrifield A, Craig JC, et al. Consistency and completeness of reported outcomes in randomized trials of primary immunosuppression in kidney transplantation. American Journal of Transplantation 2013;13(11):2892‐901. [MEDLINE: ] - PubMed
Meier‐Kriesche 2004
-
- Meier‐Kriesche HU, Schold JD, Srinvias TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. American Journal of Transplantation 2004;4(3):378‐83. [MEDLINE: ] - PubMed
Moher 2009
-
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Annals of Internal Medicine 2009;151(4):264‐9. [MEDLINE: ] - PubMed
Nankivell 2003
-
- Nankivell B, Borrows R, Fung C, O'Connell P, Allen R, Chapman J. The natural history of chronic allograft nephropathy. New England Journal of Medicine 2003;349(24):2326‐33. [MEDLINE: ] - PubMed
OPTN 2013
-
- Organ Procurement & Transplantation Network. optn.transplant.hrsa.gov/converge/data/ (accessed 11 November 2014).
Rao 2009
-
- Rao PS, Ojo A. The alphabet soup of kidney transplantation: SCD, DCD, ECD‐‐fundamentals for the practicing nephrologist. Clinical Journal of The American Society of Nephrology: CJASN 2009;4(11):1827‐31. [MEDLINE: ] - PubMed
Sayegh 1998
-
- Sayegh M, Turka L. The role of T‐cell costimulatory activation pathways in transplant rejection. New England Journal of Medicine 1998;338(25):1813‐21. [MEDLINE: ] - PubMed
SRTR 2010
-
- Scientific Registry of Transplant Recipients. 2010 Annual Report Data. srtr.transplant.hrsa.gov/annual_reports/2010/chapter_index.htm (accessed 11 November 2014).
Vanrenterghem 2008
-
- Vanrenterghem Y, Claes K, Montagnino G, Fieuws S, Maes B, Villa M, et al. Risk factors for cardiovascular events after successful renal transplantation. Transplantation 2008;85(2):209‐16. [MEDLINE: ] - PubMed
Vincenti 2007
-
- Vincenti F, Friman S, Scheurmann E, Rostaing L, Jenssen T, Campistol J, et al. Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. American Journal of Transplantation 2007;7(6):1506‐14. [MEDLINE: ] - PubMed
Watson 2005
-
- Watson CJ, Firth J, Williams P, Bradley J, Pritchard N, Chaudhry A, et al. A randomized controlled trial of late conversion from CNI‐based to sirolimus‐based immunosuppression following renal transplantation. American Journal of Transplantation 2005;5(10):2496‐503. [MEDLINE: ] - PubMed
Webster 2005
Webster 2009
-
- Webster AC, Wong G, Craig JC, Chapman JR. Managing cancer risk and decision making after kidney transplantation. American Journal of Transplantation 2008;8(11):2185‐91. [MEDLINE: ] - PubMed
White 2010
-
- White CA, Siegal D, Akbari A, Knoll GA. Use of kidney function end points in kidney transplant trials: a systematic review. American Journal of Kidney Diseases 2001;56(6):1140‐57. [MEDLINE: ] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

