Organelle-based aggregation and retention of damaged proteins in asymmetrically dividing cells
- PMID: 25417105
- PMCID: PMC6726438
- DOI: 10.1016/j.cell.2014.09.026
Organelle-based aggregation and retention of damaged proteins in asymmetrically dividing cells
Abstract
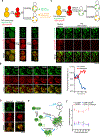
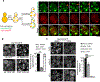
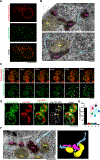
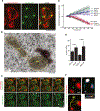
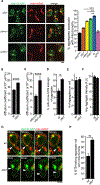
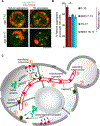
Aggregation of damaged or misfolded proteins is a protective mechanism against proteotoxic stress, abnormalities of which underlie many aging-related diseases. Here, we show that in asymmetrically dividing yeast cells, aggregation of cytosolic misfolded proteins does not occur spontaneously but requires new polypeptide synthesis and is restricted to the surface of ER, which harbors the majority of active translation sites. Protein aggregates formed on ER are frequently also associated with or are later captured by mitochondria, greatly constraining aggregate mobility. During mitosis, aggregates are tethered to well-anchored maternal mitochondria, whereas mitochondria acquired by the bud are largely free of aggregates. Disruption of aggregate-mitochondria association resulted in increased mobility and leakage of mother-accumulated aggregates into the bud. Cells with advanced replicative age exhibit gradual decline of aggregates-mitochondria association, likely contributing to their diminished ability to rejuvenate through asymmetric cell division.
Figures

References
-
- Arrasate M, Mitra S, Schweitzer ES, Segal MR, and Finkbeiner S (2004). Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature 431, 805–810. - PubMed
-
- Böckler S, and Westermann B (2014). Mitochondrial ER contacts are crucial for mitophagy in yeast. Dev. Cell 28, 450–458. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

