Spatial map of human T cell compartmentalization and maintenance over decades of life
- PMID: 25417158
- PMCID: PMC4243051
- DOI: 10.1016/j.cell.2014.10.026
Spatial map of human T cell compartmentalization and maintenance over decades of life
Abstract
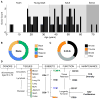
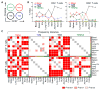
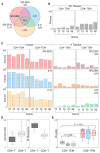
Mechanisms for human memory T cell differentiation and maintenance have largely been inferred from studies of peripheral blood, though the majority of T cells are found in lymphoid and mucosal sites. We present here a multidimensional, quantitative analysis of human T cell compartmentalization and maintenance over six decades of life in blood, lymphoid, and mucosal tissues obtained from 56 individual organ donors. Our results reveal that the distribution and tissue residence of naive, central, and effector memory, and terminal effector subsets is contingent on both their differentiation state and tissue localization. Moreover, T cell homeostasis driven by cytokine or TCR-mediated signals is different in CD4+ or CD8+ T cell lineages, varies with their differentiation stage and tissue localization, and cannot be inferred from blood. Our data provide an unprecedented spatial and temporal map of human T cell compartmentalization and maintenance, supporting distinct pathways for human T cell fate determination and homeostasis.
Figures




References
-
- Ahmed R, Bevan MJ, Reiner SL, Fearon DT. The precursors of memory: models and controversies. Nat Rev Immunol. 2009;9:662–668. - PubMed
-
- Campbell JJ, Murphy KE, Kunkel EJ, Brightling CE, Soler D, Shen Z, Boisvert J, Greenberg HB, Vierra MA, Goodman SB, et al. CCR7 expression and memory T cell diversity in humans. J Immunol. 2001;166:877–884. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

