Allosteric communication in the dynein motor domain
- PMID: 25417161
- PMCID: PMC4269335
- DOI: 10.1016/j.cell.2014.10.018
Allosteric communication in the dynein motor domain
Abstract
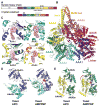
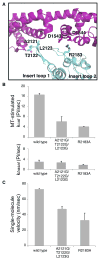
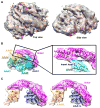
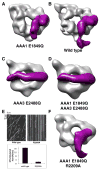
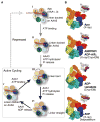
Dyneins power microtubule motility using ring-shaped, AAA-containing motor domains. Here, we report X-ray and electron microscopy (EM) structures of yeast dynein bound to different ATP analogs, which collectively provide insight into the roles of dynein's two major ATPase sites, AAA1 and AAA3, in the conformational change mechanism. ATP binding to AAA1 triggers a cascade of conformational changes that propagate to all six AAA domains and cause a large movement of the "linker," dynein's mechanical element. In contrast to the role of AAA1 in driving motility, nucleotide transitions in AAA3 gate the transmission of conformational changes between AAA1 and the linker, suggesting that AAA3 acts as a regulatory switch. Further structural and mutational studies also uncover a role for the linker in regulating the catalytic cycle of AAA1. Together, these results reveal how dynein's two major ATP-binding sites initiate and modulate conformational changes in the motor domain during motility.
Figures
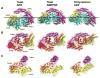

References
-
- Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, Terwilliger TC. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr D Biol Crystallogr. 2002;58:1948–1954. - PubMed
-
- Burgess SA, Knight PJ. Is the dynein motor a winch? Curr Opin Struct Biol. 2004;14:138–146. - PubMed
-
- Burgess SA, Walker ML, Sakakibara H, Knight PJ, Oiwa K. Dynein structure and power stroke. Nature. 2003;421:715–718. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

