ATRX directs binding of PRC2 to Xist RNA and Polycomb targets
- PMID: 25417162
- PMCID: PMC4379047
- DOI: 10.1016/j.cell.2014.10.019
ATRX directs binding of PRC2 to Xist RNA and Polycomb targets
Erratum in
- Cell. 2014 Nov 20;159(5):1228
- Cell. 2015 Jan 29;160(3):568-9
Abstract
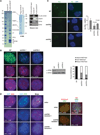
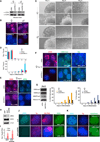
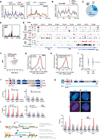
X chromosome inactivation (XCI) depends on the long noncoding RNA Xist and its recruitment of Polycomb Repressive Complex 2 (PRC2). PRC2 is also targeted to other sites throughout the genome to effect transcriptional repression. Using XCI as a model, we apply an unbiased proteomics approach to isolate Xist and PRC2 regulators and identified ATRX. ATRX unexpectedly functions as a high-affinity RNA-binding protein that directly interacts with RepA/Xist RNA to promote loading of PRC2 in vivo. Without ATRX, PRC2 cannot load onto Xist RNA nor spread in cis along the X chromosome. Moreover, epigenomic profiling reveals that genome-wide targeting of PRC2 depends on ATRX, as loss of ATRX leads to spatial redistribution of PRC2 and derepression of Polycomb responsive genes. Thus, ATRX is a required specificity determinant for PRC2 targeting and function.
Figures




Similar articles
-
The Xist RNA-PRC2 complex at 20-nm resolution reveals a low Xist stoichiometry and suggests a hit-and-run mechanism in mouse cells.Proc Natl Acad Sci U S A. 2015 Aug 4;112(31):E4216-25. doi: 10.1073/pnas.1503690112. Epub 2015 Jul 20. Proc Natl Acad Sci U S A. 2015. PMID: 26195790 Free PMC article.
-
Jarid2 Is Implicated in the Initial Xist-Induced Targeting of PRC2 to the Inactive X Chromosome.Mol Cell. 2014 Jan 23;53(2):301-16. doi: 10.1016/j.molcel.2014.01.002. Mol Cell. 2014. PMID: 24462204
-
The Xist lncRNA interacts directly with SHARP to silence transcription through HDAC3.Nature. 2015 May 14;521(7551):232-6. doi: 10.1038/nature14443. Epub 2015 Apr 27. Nature. 2015. PMID: 25915022 Free PMC article.
-
Noncoding RNA and Polycomb recruitment.RNA. 2013 Apr;19(4):429-42. doi: 10.1261/rna.037598.112. Epub 2013 Feb 19. RNA. 2013. PMID: 23431328 Free PMC article. Review.
-
The many faces of Polycomb regulation by RNA.Curr Opin Genet Dev. 2020 Apr;61:53-61. doi: 10.1016/j.gde.2020.02.023. Epub 2020 May 11. Curr Opin Genet Dev. 2020. PMID: 32403014 Free PMC article. Review.
Cited by
-
Sexual dimorphism in cancer.Nat Rev Cancer. 2016 May;16(5):330-9. doi: 10.1038/nrc.2016.30. Epub 2016 Apr 15. Nat Rev Cancer. 2016. PMID: 27079803 Review.
-
ATRX loss induces telomere dysfunction and necessitates induction of alternative lengthening of telomeres during human cell immortalization.EMBO J. 2019 Oct 1;38(19):e96659. doi: 10.15252/embj.201796659. Epub 2019 Aug 27. EMBO J. 2019. PMID: 31454099 Free PMC article.
-
RNA exosome drives early B cell development via noncoding RNA processing mechanisms.Sci Immunol. 2022 Jun 3;7(72):eabn2738. doi: 10.1126/sciimmunol.abn2738. Epub 2022 Jun 3. Sci Immunol. 2022. PMID: 35658015 Free PMC article.
-
Long noncoding RNA VPS9D1-AS1 promotes esophageal squamous cell carcinoma progression via the Wnt/β-catenin signaling pathway.J Cancer. 2021 Oct 2;12(22):6894-6904. doi: 10.7150/jca.54556. eCollection 2021. J Cancer. 2021. PMID: 34659577 Free PMC article.
-
G-quadruplex DNA drives genomic instability and represents a targetable molecular abnormality in ATRX-deficient malignant glioma.Nat Commun. 2019 Feb 26;10(1):943. doi: 10.1038/s41467-019-08905-8. Nat Commun. 2019. PMID: 30808951 Free PMC article.
References
-
- Brown CJ, Hendrich BD, Rupert JL, Lafreniere RG, Xing Y, Lawrence J, Willard HF. The human XIST gene: analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell. 1992;71:527–542. - PubMed
-
- Cardoso C, Timsit S, Villard L, Khrestchatisky M, Fontés M, Colleaux L. Specific interaction between the XNP/ATR-X gene product and the SET domain of the human EZH2 protein. Hum. Mol. Genet. 1998;7:679–684. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

