At the centre: influenza A virus ribonucleoproteins
- PMID: 25417656
- PMCID: PMC5619696
- DOI: 10.1038/nrmicro3367
At the centre: influenza A virus ribonucleoproteins
Abstract
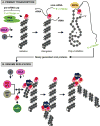
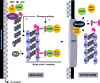
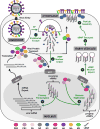
Influenza A viral ribonucleoprotein (vRNP) complexes comprise the eight genomic negative-sense RNAs, each of which is bound to multiple copies of the vRNP and a trimeric viral polymerase complex. The influenza virus life cycle centres on the vRNPs, which in turn rely on host cellular processes to carry out functions that are necessary for the successful completion of the virus life cycle. In this Review, we discuss our current knowledge about vRNP trafficking within host cells and the function of these complexes in the context of the virus life cycle, highlighting how structure contributes to function and the crucial interactions with host cell pathways, as well as on the information gaps that remain. An improved understanding of how vRNPs use host cell pathways is essential to identify mechanisms of virus pathogenicity, host adaptation and, ultimately, new targets for antiviral intervention.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures



References
-
- WHO. Influenza (Seasonal) Fact sheet N°211. 2009 < http://www.who.int/mediacentre/factsheets/fs211/en/index.html>.
-
- Simonsen L. The global impact of influenza on morbidity and mortality. Vaccine. 1999;17(Suppl 1):S3–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

