Engineering the control of mosquito-borne infectious diseases
- PMID: 25418061
- PMCID: PMC4282146
- DOI: 10.1186/s13059-014-0535-7
Engineering the control of mosquito-borne infectious diseases
Abstract
Recent advances in genetic engineering are bringing new promise for controlling mosquito populations that transmit deadly pathogens. Here we discuss past and current efforts to engineer mosquito strains that are refractory to disease transmission or are suitable for suppressing wild disease-transmitting populations.
Figures
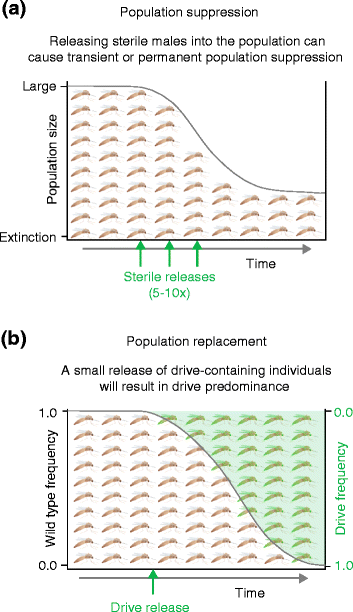
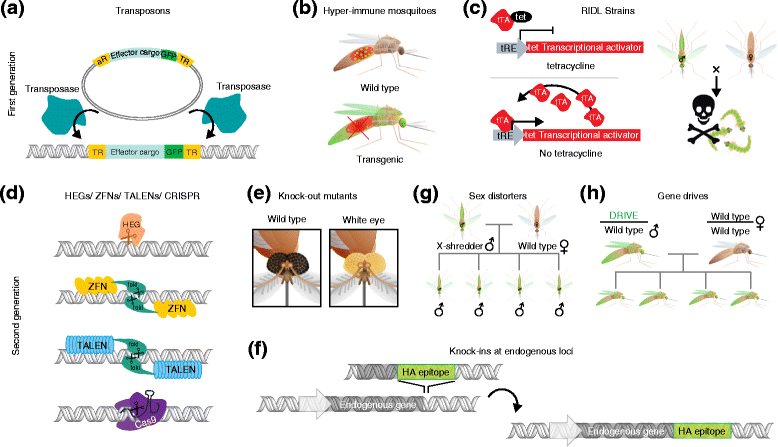
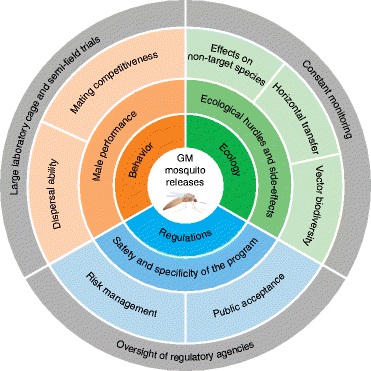
References
-
- World Health Organization . The World Malaria Report 2013. 2013.
-
- Taylor B. Changes in the feeding behaviour of a malaria vector, Anopheles farauti Lav., following use of DDT as a residual spray in houses in the British Solomon Islands Protectorate. Trans R Entomol Soc Lond. 1975;127:277–292. doi: 10.1111/j.1365-2311.1975.tb00576.x. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

