Translational toxicology and rescue strategies of the hERG channel dysfunction: biochemical and molecular mechanistic aspects
- PMID: 25418379
- PMCID: PMC4261120
- DOI: 10.1038/aps.2014.101
Translational toxicology and rescue strategies of the hERG channel dysfunction: biochemical and molecular mechanistic aspects
Abstract
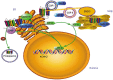
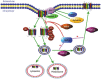
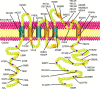
The human ether-à-go-go related gene (hERG) potassium channel is an obligatory anti-target for drug development on account of its essential role in cardiac repolarization and its close association with arrhythmia. Diverse drugs have been removed from the market owing to their inhibitory activity on the hERG channel and their contribution to acquired long QT syndrome (LQTS). Moreover, mutations that cause hERG channel dysfunction may induce congenital LQTS. Recently, an increasing number of biochemical and molecular mechanisms underlying hERG-associated LQTS have been reported. In fact, numerous potential biochemical and molecular rescue strategies are hidden within the biogenesis and regulating network. So far, rescue strategies of hERG channel dysfunction and LQTS mainly include activators, blockers, and molecules that interfere with specific links and other mechanisms. The aim of this review is to discuss the rescue strategies based on hERG channel toxicology from the biochemical and molecular perspectives.
Figures

References
-
- Vandenberg JI, Perry MD, Perrin MJ, Mann SA, Ke Y, Hill AP. hERG K+ channels: structure, function, and clinical significance. Physiol Rev. 2012;92:1393–478. - PubMed
-
- Sanguinetii MC, Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature. 2006;440:463–9. - PubMed
-
- Sanguinetti MC, Jiang CG, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the Ikr potassium channel. Cell. 1995;81:299–307. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

