Transmembrane Inhibitor of RICTOR/mTORC2 in Hematopoietic Progenitors
- PMID: 25418727
- PMCID: PMC4235746
- DOI: 10.1016/j.stemcr.2014.08.011
Transmembrane Inhibitor of RICTOR/mTORC2 in Hematopoietic Progenitors
Abstract
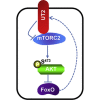
Central to cellular proliferative, survival, and metabolic responses is the serine/threonine kinase mTOR, which is activated in many human cancers. mTOR is present in distinct complexes that are either modulated by AKT (mTORC1) or are upstream and regulatory of it (mTORC2). Governance of mTORC2 activity is poorly understood. Here, we report a transmembrane molecule in hematopoietic progenitor cells that physically interacts with and inhibits RICTOR, an essential component of mTORC2. Upstream of mTORC2 (UT2) negatively regulates mTORC2 enzymatic activity, reducing AKT(S473), PKCα, and NDRG1 phosphorylation and increasing FOXO transcriptional activity in an mTORC2-dependent manner. Modulating UT2 levels altered animal survival in a T cell acute lymphoid leukemia (T-ALL) model that is known to be mTORC2 sensitive. These studies identify an inhibitory component upstream of mTORC2 in hematopoietic cells that can reduce mortality from NOTCH-induced T-ALL. A transmembrane inhibitor of mTORC2 may provide an attractive target to affect this critical cell regulatory pathway.
Figures



References
-
- Blackburn J.S., Liu S., Wilder J.L., Dobrinski K.P., Lobbardi R., Moore F.E., Martinez S.A., Chen E.Y., Lee C., Langenau D.M. Clonal evolution enhances leukemia-propagating cell frequency in T cell acute lymphoblastic leukemia through Akt/mTORC1 pathway activation. Cancer Cell. 2014;25:366–378. - PMC - PubMed
-
- Brunet A., Bonni A., Zigmond M.J., Lin M.Z., Juo P., Hu L.S., Anderson M.J., Arden K.C., Blenis J., Greenberg M.E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. - PubMed
-
- García-Martínez J.M., Alessi D.R. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1) Biochem. J. 2008;416:375–385. - PubMed
-
- Guertin D.A., Stevens D.M., Thoreen C.C., Burds A.A., Kalaany N.Y., Moffat J., Brown M., Fitzgerald K.J., Sabatini D.M. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev. Cell. 2006;11:859–871. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

