A dominant-negative isoform of IKAROS expands primitive normal human hematopoietic cells
- PMID: 25418728
- PMCID: PMC4235152
- DOI: 10.1016/j.stemcr.2014.09.006
A dominant-negative isoform of IKAROS expands primitive normal human hematopoietic cells
Abstract
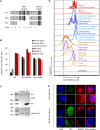
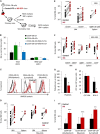
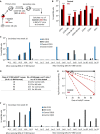
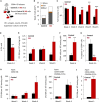
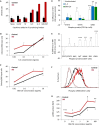
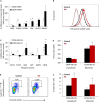
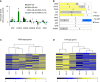
Disrupted IKAROS activity is a recurrent feature of some human leukemias, but effects on normal human hematopoietic cells are largely unknown. Here, we used lentivirally mediated expression of a dominant-negative isoform of IKAROS (IK6) to block normal IKAROS activity in primitive human cord blood cells and their progeny. This produced a marked (10-fold) increase in serially transplantable multipotent IK6(+) cells as well as increased outputs of normally differentiating B cells and granulocytes in transplanted immunodeficient mice, without producing leukemia. Accompanying T/natural killer (NK) cell outputs were unaltered, and erythroid and platelet production was reduced. Mechanistically, IK6 specifically increased human granulopoietic progenitor sensitivity to two growth factors and activated CREB and its targets (c-FOS and Cyclin B1). In more primitive human cells, IK6 prematurely initiated a B cell transcriptional program without affecting the hematopoietic stem cell-associated gene expression profile. Some of these effects were species specific, thus identifying novel roles of IKAROS in regulating normal human hematopoietic cells.
Figures
References
-
- Aghaeepour N., Chattopadhyay P.K., Ganesan A., O’Neill K., Zare H., Jalali A., Hoos H.H., Roederer M., Brinkman R.R. Early immunologic correlates of HIV protection can be identified from computational analysis of complex multivariate T-cell flow cytometry assays. Bioinformatics. 2012;28:1009–1016. - PMC - PubMed
-
- Billerbeck E., Barry W.T., Mu K., Dorner M., Rice C.M., Ploss A. Development of human CD4+FoxP3+ regulatory T cells in human stem cell factor-, granulocyte-macrophage colony-stimulating factor-, and interleukin-3-expressing NOD-SCID IL2Rγ(null) humanized mice. Blood. 2011;117:3076–3086. - PMC - PubMed
-
- Carbonaro D.A., Jin X., Petersen D., Wang X., Dorey F., Kil K.S., Aldrich M., Blackburn M.R., Kellems R.E., Kohn D.B. In vivo transduction by intravenous injection of a lentiviral vector expressing human ADA into neonatal ADA gene knockout mice: a novel form of enzyme replacement therapy for ADA deficiency. Mol. Ther. 2006;13:1110–1120. - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

