Efficient utilization of EBUS-TBNA samples for both diagnosis and molecular analyses
- PMID: 25419143
- PMCID: PMC4234164
- DOI: 10.2147/OTT.S72974
Efficient utilization of EBUS-TBNA samples for both diagnosis and molecular analyses
Abstract
Background: The assessment of an increasing number of molecular markers is becoming a standard requirement from endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) specimens. However, it is unclear how many needle passes should be performed and the amount of lung cancer cells that should be sent for molecular analyses. The objective of this study was to determine if it is feasible to divide the material obtained by EBUS-TBNA to allow for molecular analysis without compromising the accuracy of mediastinal staging.
Objective: We aimed to determine if dividing EBUS-TBNA specimens has a negative impact on either histopathological diagnosis or molecular analysis.
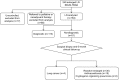
Methods: EBUS-TBNA was performed in 249 enlarged lymph nodes. Negative or ambiguous histopathological results were confirmed by surgical means and clinical follow-up over 6 months. The tissue obtained by EBUS-TBNA was placed onto a glass slide and divided for histopathological workup and molecular analysis. The number of passes was recorded. Both the accuracy of the mediastinal lymph node staging and the applicability of the sample division for molecular analysis were assessed.
Results: Each lymph node was punctured an average of 3.18 times and division of the obtained material for diagnosis and molecular analysis was feasible in all cases. The sensitivity and accuracy of the mediastinal lymph node staging were 96.6% and 97.6%, respectively. A cytokeratin (CK)-19-mRNA concentration-based molecular test was feasible in 74.1% of cases.
Conclusion: Dividing EBUS-TBNA samples for both histopathological diagnosis and molecular testing is feasible and does not compromise the accuracy of mediastinal staging. This method may be an alternative to taking additional needle passes for molecular analyses.
Keywords: CK-19-mRNA; lung cancer; lymph node sampling; molecular marker.
Figures

References
-
- Varela-Lema L, Fernández-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: a systematic review. Eur Respir J. 2009;33(5):1156–1164. - PubMed
-
- Yasufuku K, Pierre A, Darling G, et al. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg. 2011;142(6):1393–1400. e1. - PubMed
-
- Nakajima T, Yasufuku K. How I do it – optimal methodology for multidirectional analysis of endobronchial ultrasound-guided transbronchial needle aspiration samples. J Thorac Oncol. 2011;6(1):203–206. - PubMed
-
- Wallace MB, Block MI, Gillanders W, et al. Accurate molecular detection of non-small cell lung cancer metastases in mediastinal lymph nodes sampled by endoscopic ultrasound-guided needle aspiration. Chest. 2005;127(2):430–437. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

