Kartogenin induces cartilage-like tissue formation in tendon-bone junction
- PMID: 25419468
- PMCID: PMC4237211
- DOI: 10.1038/boneres.2014.8
Kartogenin induces cartilage-like tissue formation in tendon-bone junction
Abstract
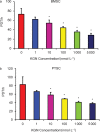
Tendon-bone junctions (TBJs) are frequently injured, especially in athletic settings. Healing of TBJ injuries is slow and is often repaired with scar tissue formation that compromises normal function. This study explored the feasibility of using kartogenin (KGN), a biocompound, to enhance the healing of injured TBJs. We first determined the effects of KGN on the proliferation and chondrogenic differentiation of rabbit bone marrow stromal cells (BMSCs) and patellar tendon stem/progenitor cells (PTSCs) in vitro. KGN enhanced cell proliferation in both cell types in a concentration-dependent manner and induced chondrogenic differentiation of stem cells, as demonstrated by high expression levels of chondrogenic markers aggrecan, collagen II and Sox-9. Besides, KGN induced the formation of cartilage-like tissues in cell cultures, as observed through the staining of abundant proteoglycans, collagen II and osteocalcin. When injected into intact rat patellar tendons in vivo, KGN induced cartilage-like tissue formation in the injected area. Similarly, when KGN was injected into experimentally injured rat Achilles TBJs, wound healing in the TBJs was enhanced, as evidenced by the formation of extensive cartilage-like tissues. These results suggest that KGN may be used as an effective cell-free clinical therapy to enhance the healing of injured TBJs.
Figures





Similar articles
-
A Novel Approach for Meniscal Regeneration Using Kartogenin-Treated Autologous Tendon Graft.Am J Sports Med. 2017 Dec;45(14):3289-3297. doi: 10.1177/0363546517721192. Epub 2017 Sep 1. Am J Sports Med. 2017. PMID: 28859517
-
A novel kartogenin-platelet-rich plasma gel enhances chondrogenesis of bone marrow mesenchymal stem cells in vitro and promotes wounded meniscus healing in vivo.Stem Cell Res Ther. 2019 Jul 8;10(1):201. doi: 10.1186/s13287-019-1314-x. Stem Cell Res Ther. 2019. PMID: 31287023 Free PMC article.
-
The combined use of kartogenin and platelet-rich plasma promotes fibrocartilage formation in the wounded rat Achilles tendon entheses.Bone Joint Res. 2017 Apr;6(4):231-244. doi: 10.1302/2046-3758.64.BJR-2017-0268.R1. Bone Joint Res. 2017. PMID: 28450316 Free PMC article.
-
Kartogenin and Its Application in Regenerative Medicine.Curr Med Sci. 2019 Feb;39(1):16-20. doi: 10.1007/s11596-019-1994-6. Epub 2019 Mar 13. Curr Med Sci. 2019. PMID: 30868486 Review.
-
Application of kartogenin for musculoskeletal regeneration.J Biomed Mater Res A. 2018 Apr;106(4):1141-1148. doi: 10.1002/jbm.a.36300. Epub 2017 Dec 10. J Biomed Mater Res A. 2018. PMID: 29164815 Review.
Cited by
-
Enhancement of rotator cuff tendon-bone healing using combined aligned electrospun fibrous membranes and kartogenin.RSC Adv. 2019 May 17;9(27):15582-15592. doi: 10.1039/c8ra09849b. eCollection 2019 May 14. RSC Adv. 2019. PMID: 35514830 Free PMC article.
-
Kartogenin hydrolysis product 4-aminobiphenyl distributes to cartilage and mediates cartilage regeneration.Theranostics. 2019 Sep 21;9(24):7108-7121. doi: 10.7150/thno.38182. eCollection 2019. Theranostics. 2019. PMID: 31695756 Free PMC article.
-
Fabrication of Injectable Kartogenin-Conjugated Composite Hydrogel with a Sustained Drug Release for Cartilage Repair.Pharmaceutics. 2023 Jul 14;15(7):1949. doi: 10.3390/pharmaceutics15071949. Pharmaceutics. 2023. PMID: 37514135 Free PMC article.
-
Investigation and Comparison of the Effect of TGF-β3, kartogenin and Avocado/Soybean Unsaponifiables on the In-vitro and In-vivo Chondrogenesis of Human Adipose-Derived Stem Cells on Fibrin Scaffold.Iran J Pharm Res. 2021 Summer;20(3):368-380. doi: 10.22037/ijpr.2020.114420.14851. Iran J Pharm Res. 2021. PMID: 34903995 Free PMC article.
-
Controlled-release hydrogel loaded with magnesium-based nanoflowers synergize immunomodulation and cartilage regeneration in tendon-bone healing.Bioact Mater. 2024 Feb 28;36:62-82. doi: 10.1016/j.bioactmat.2024.02.024. eCollection 2024 Jun. Bioact Mater. 2024. PMID: 38440323 Free PMC article.
References
-
- Hibino N, Hamada Y, Sairyo K, Yukata K, Sano T, Yasui N. Callus formation during healing of the repaired tendon-bone junction. A rat experimental model. J Bone Joint Surg Br 2007; 89: 1539–1544. - PubMed
-
- Raspanti M, Strocchi R, de Pasquale V, Martini D, Montanari C, Ruggeri A. Structure and ultrastructure of the bone/ligament junction. Ital J Anat Embryol 1996; 101: 97–105. - PubMed
-
- Woo S, Manynard J, Butler D. Ligament, tendon, and joint capsule insertions to bone. In: Woo S Buckwalter J (ed.) Injury and Repair of the Musculoskeletal Soft Tissues. Park Ridge, IL: American Academy of Orthopaedic Surgeons Symposium, 1988: 129–166.
-
- Nebelung W, Becker R, Urbach D, Ropke M, Roessner A. Histological findings of tendon–bone healing following anterior cruciate ligament reconstruction with hamstring grafts. Arch Orthop Trauma Surg 2003; 123: 158–163. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

