Influence of the timing of antiretroviral therapy on the potential for normalization of immune status in human immunodeficiency virus 1-infected individuals
- PMID: 25419650
- PMCID: PMC4286496
- DOI: 10.1001/jamainternmed.2014.4010
Influence of the timing of antiretroviral therapy on the potential for normalization of immune status in human immunodeficiency virus 1-infected individuals
Abstract
Importance: In individuals with human immunodeficiency virus 1 (HIV-1) infection who are receiving antiretroviral therapy (ART), factors that promote full immune recovery are not well characterized.
Objective: To investigate the influence of the timing of ART relative to HIV-1 infection on normalization of CD4+ T-cell counts, AIDS risk, and immune function.
Design, setting, and participants: Participants in the observational US Military HIV Natural History Study with documented estimated dates of seroconversion (EDS) who achieved virologic suppression with ART were evaluated. Markers indicative of immune activation, dysfunction, and responsiveness were determined. Responses to hepatitis B virus (HBV) vaccine, an indicator of in vivo immune function, were also assessed. The timing of ART was indexed to the EDS and/or entry into the cohort. The CD4+ counts in HIV-1-uninfected populations were surveyed.
Main outcomes and measures: Normalization of CD4+ counts to 900 cells/μL or higher, AIDS development, HBV vaccine response, as well as T-cell activation, dysfunction, and responsiveness.
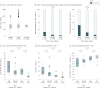
Results: The median CD4+ count in HIV-1-uninfected populations was approximately 900 cells/μL. Among 1119 HIV-1-infected participants, CD4+ normalization was achieved in 38.4% vs 28.3% of those initiating ART within 12 months vs after 12 months from the EDS (P = .001). Incrementally higher CD4+ recovery (<500, 500-899, and ≥900 cells/μL) was associated with stepwise decreases in AIDS risk and reversion of markers of immune activation, dysfunction, and responsiveness to levels approximating those found in HIV-1-uninfected persons. Participants with CD4+ counts of 500 cells/μL or higher at study entry (adjusted odds ratio [aOR], 2.00; 95% CI, 1.51-2.64; P < .001) or ART initiation (aOR, 4.08; 95% CI, 3.14-5.30; P < .001) had significantly increased CD4+ normalization rates compared with other participants. However, even among individuals with a CD4+ count of 500 cells/μL or higher at both study entry and before ART, the odds of CD4+ normalization were 80% lower in those initiating ART after 12 months from the EDS and study entry (aOR, 0.20; 95% CI, 0.07-0.53; P = 001). Initiation of ART within 12 months of EDS vs later was associated with a significantly lower risk of AIDS (7.8% vs 15.3%; P = .002), reduced T-cell activation (percent CD4+HLA-DR+ effector memory T cells, 12.0% vs 15.6%; P = .03), and increased responsiveness to HBV vaccine (67.9% vs 50.9%; P = .07).
Conclusions and relevance: Deferral of ART beyond 12 months of the EDS diminishes the likelihood of restoring immunologic health in HIV-1-infected individuals.
Conflict of interest statement
Figures


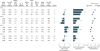

Comment in
-
Defining success with antiretroviral therapy.JAMA Intern Med. 2015 Jan;175(1):99-100. doi: 10.1001/jamainternmed.2014.4004. JAMA Intern Med. 2015. PMID: 25419970 No abstract available.
Similar articles
-
Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy.N Engl J Med. 2013 Jan 17;368(3):218-30. doi: 10.1056/NEJMoa1110187. N Engl J Med. 2013. PMID: 23323898 Free PMC article.
-
Determinants of suboptimal CD4+ T cell recovery after antiretroviral therapy initiation in a prospective cohort of acute HIV-1 infection.J Int AIDS Soc. 2020 Sep;23(9):e25585. doi: 10.1002/jia2.25585. J Int AIDS Soc. 2020. PMID: 32949118 Free PMC article.
-
Thirty-day postoperative mortality among individuals with HIV infection receiving antiretroviral therapy and procedure-matched, uninfected comparators.JAMA Surg. 2015 Apr;150(4):343-51. doi: 10.1001/jamasurg.2014.2257. JAMA Surg. 2015. PMID: 25714794 Free PMC article.
-
From CD4-Based Initiation to Treating All HIV-Infected Adults Immediately: An Evidence-Based Meta-analysis.Front Immunol. 2018 Feb 13;9:212. doi: 10.3389/fimmu.2018.00212. eCollection 2018. Front Immunol. 2018. PMID: 29487595 Free PMC article.
-
Recovery of the immune system with antiretroviral therapy: the end of opportunism?JAMA. 1998 Jul 1;280(1):72-7. doi: 10.1001/jama.280.1.72. JAMA. 1998. PMID: 9660367
Cited by
-
No Evidence for an Association of HIV and Antiviral Treatment With Changes in Framingham Cardiovascular Risk Score in the Ndlovu Cohort Study.J Am Heart Assoc. 2024 Jan 16;13(2):e029637. doi: 10.1161/JAHA.123.029637. Epub 2024 Jan 12. J Am Heart Assoc. 2024. PMID: 38214319 Free PMC article.
-
Antiretroviral Initiation at ≥800 CD4+ Cells/mm3 Associated With Lower Human Immunodeficiency Virus Reservoir Size.Clin Infect Dis. 2022 Nov 14;75(10):1781-1791. doi: 10.1093/cid/ciac249. Clin Infect Dis. 2022. PMID: 35396591 Free PMC article.
-
Transmitted drug resistance in patients with acute/recent HIV infection in Brazil.Braz J Infect Dis. 2017 Jul-Aug;21(4):396-401. doi: 10.1016/j.bjid.2017.03.013. Epub 2017 May 21. Braz J Infect Dis. 2017. PMID: 28539254 Free PMC article.
-
The benefit of immediate compared with deferred antiretroviral therapy on CD4+ cell count recovery in early HIV infection.AIDS. 2019 Jul 1;33(8):1335-1344. doi: 10.1097/QAD.0000000000002219. AIDS. 2019. PMID: 31157663 Free PMC article. Clinical Trial.
-
Influence of immune activation on the risk of allograft rejection in human immunodeficiency virus-infected kidney transplant recipients.Transpl Immunol. 2016 Sep;38:40-43. doi: 10.1016/j.trim.2016.06.001. Epub 2016 Jun 11. Transpl Immunol. 2016. PMID: 27297667 Free PMC article.
References
-
- Guihot A, Bourgarit A, Carcelain G, Autran B. Immune reconstitution after a decade of combined antiretroviral therapies for human immunodeficiency virus. Trends Immunol. 2011;32(3):131–137. - PubMed
-
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Health Organization website. http://www.who.int/hiv/pub/guidelines/arv2013. Published June 2013. Accessed June 9, 2014.
Publication types
MeSH terms
Substances
Grants and funding
- P01 AI080193/AI/NIAID NIH HHS/United States
- R37AI046326/AI/NIAID NIH HHS/United States
- R21 AI077304/AI/NIAID NIH HHS/United States
- R37 AI046326/AI/NIAID NIH HHS/United States
- UM1 AI069432/AI/NIAID NIH HHS/United States
- R01 AI043279/AI/NIAID NIH HHS/United States
- U01 AI069432/AI/NIAID NIH HHS/United States
- U01 AI043638/AI/NIAID NIH HHS/United States
- DP1 DA034978/DA/NIDA NIH HHS/United States
- P30 MH062512/MH/NIMH NIH HHS/United States
- UL1 TR001120/TR/NCATS NIH HHS/United States
- U19 AI096113/AI/NIAID NIH HHS/United States
- R24 AI106039/AI/NIAID NIH HHS/United States
- IP1 CX000875/CX/CSRD VA/United States
- R01 MH097520/MH/NIMH NIH HHS/United States
- Y01 AI005072/AI/NIAID NIH HHS/United States
- P01 AI074621/AI/NIAID NIH HHS/United States
- R01 MH100974/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

