Employing a gain-of-function factor IX variant R338L to advance the efficacy and safety of hemophilia B human gene therapy: preclinical evaluation supporting an ongoing adeno-associated virus clinical trial
- PMID: 25419787
- PMCID: PMC4326268
- DOI: 10.1089/hum.2014.106
Employing a gain-of-function factor IX variant R338L to advance the efficacy and safety of hemophilia B human gene therapy: preclinical evaluation supporting an ongoing adeno-associated virus clinical trial
Abstract
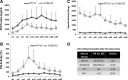
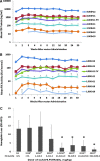
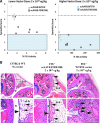
Vector capsid dose-dependent inflammation of transduced liver has limited the ability of adeno-associated virus (AAV) factor IX (FIX) gene therapy vectors to reliably convert severe to mild hemophilia B in human clinical trials. These trials also identified the need to understand AAV neutralizing antibodies and empty AAV capsids regarding their impact on clinical success. To address these safety concerns, we have used a scalable manufacturing process to produce GMP-grade AAV8 expressing the FIXR338L gain-of-function variant with minimal (<10%) empty capsid and have performed comprehensive dose-response, biodistribution, and safety evaluations in clinically relevant hemophilia models. The scAAV8.FIXR338L vector produced greater than 6-fold increased FIX specific activity compared with wild-type FIX and demonstrated linear dose responses from doses that produced 2-500% FIX activity, associated with dose-dependent hemostasis in a tail transection bleeding challenge. More importantly, using a bleeding model that closely mimics the clinical morbidity of hemophilic arthropathy, mice that received the scAAV8.FIXR338L vector developed minimal histopathological findings of synovitis after hemarthrosis, when compared with mice that received identical doses of wild-type FIX vector. Hemostatically normal mice (n=20) and hemophilic mice (n=88) developed no FIX antibodies after peripheral intravenous vector delivery. No CD8(+) T cell liver infiltrates were observed, despite the marked tropism of scAAV8.FIXR338L for the liver in a comprehensive biodistribution evaluation (n=60 animals). With respect to the role of empty capsids, we demonstrated that in vivo FIXR338L expression was not influenced by the presence of empty AAV particles, either in the presence or absence of various titers of AAV8-neutralizing antibodies. Necropsy of FIX(-/-) mice 8-10 months after vector delivery revealed no microvascular or macrovascular thrombosis in mice expressing FIXR338L (plasma FIX activity, 100-500%). These preclinical studies demonstrate a safety:efficacy profile supporting an ongoing phase 1/2 human clinical trial of the scAAV8.FIXR338L vector (designated BAX335).
Figures
References
-
- Manno CS, Pierce GF, Arruda VR, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med 2006;12:342–347 - PubMed
-
- Vandendriessche T, Thorrez L, Acosta-Sanchez A, et al. Efficacy and safety of adeno-associated viral vectors based on serotype 8 and 9 vs. lentiviral vectors for hemophilia B gene therapy. J Thromb Haemost 2007;5:16–24 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

