SOX17 links gut endoderm morphogenesis and germ layer segregation
- PMID: 25419850
- PMCID: PMC4250291
- DOI: 10.1038/ncb3070
SOX17 links gut endoderm morphogenesis and germ layer segregation
Abstract
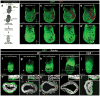
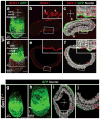
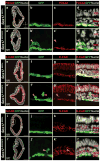
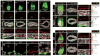
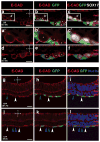
Gastrulation leads to three germ layers--ectoderm, mesoderm and endoderm--that are separated by two basement membranes. In the mouse embryo, the emergent gut endoderm results from the widespread intercalation of cells of two distinct origins: pluripotent epiblast-derived definitive endoderm (DE) and extra-embryonic visceral endoderm (VE). Here we image the trajectory of prospective DE cells before intercalating into the VE epithelium. We show that the transcription factor SOX17, which is activated in prospective DE cells before intercalation, is necessary for gut endoderm morphogenesis and the assembly of the basement membrane that separates gut endoderm from mesoderm. Our results mechanistically link gut endoderm morphogenesis and germ layer segregation, two central and conserved features of gastrulation.
Figures



Comment in
-
Gut endoderm takes flight from the wings of mesoderm.Nat Cell Biol. 2014 Dec;16(12):1128-9. doi: 10.1038/ncb3077. Nat Cell Biol. 2014. PMID: 25434463
References
-
- Arnold SJ, Robertson EJ. Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nat Rev Mol Cell Biol. 2009;10:91–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

