A learning-based approach to artificial sensory feedback leads to optimal integration
- PMID: 25420067
- PMCID: PMC4282864
- DOI: 10.1038/nn.3883
A learning-based approach to artificial sensory feedback leads to optimal integration
Abstract
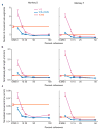
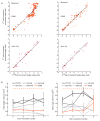
Proprioception-the sense of the body's position in space-is important to natural movement planning and execution and will likewise be necessary for successful motor prostheses and brain-machine interfaces (BMIs). Here we demonstrate that monkeys were able to learn to use an initially unfamiliar multichannel intracortical microstimulation signal, which provided continuous information about hand position relative to an unseen target, to complete accurate reaches. Furthermore, monkeys combined this artificial signal with vision to form an optimal, minimum-variance estimate of relative hand position. These results demonstrate that a learning-based approach can be used to provide a rich artificial sensory feedback signal, suggesting a new strategy for restoring proprioception to patients using BMIs, as well as a powerful new tool for studying the adaptive mechanisms of sensory integration.
Figures


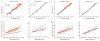

References
-
- van Beers RJ, Sittig AC, Gon JJ. Integration of proprioceptive and visual position–information: An experimentally supported model. J Neurophysiol. 1999;81:1355–1364. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

