International regulatory landscape and integration of corrective genome editing into in vitro fertilization
- PMID: 25420886
- PMCID: PMC4251934
- DOI: 10.1186/1477-7827-12-108
International regulatory landscape and integration of corrective genome editing into in vitro fertilization
Abstract
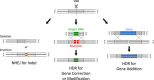
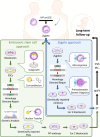
Genome editing technology, including zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and clustered regularly interspaced short palindromic repeat (CRISPR)/Cas, has enabled far more efficient genetic engineering even in non-human primates. This biotechnology is more likely to develop into medicine for preventing a genetic disease if corrective genome editing is integrated into assisted reproductive technology, represented by in vitro fertilization. Although rapid advances in genome editing are expected to make germline gene correction feasible in a clinical setting, there are many issues that still need to be addressed before this could occur. We herein examine current status of genome editing in mammalian embryonic stem cells and zygotes and discuss potential issues in the international regulatory landscape regarding human germline gene modification. Moreover, we address some ethical and social issues that would be raised when each country considers whether genome editing-mediated germline gene correction for preventive medicine should be permitted.
Figures

References
-
- Billings PR, Hubbard R, Newman SA. Human germline gene modification: a dissent. Lancet. 1999;353(9167):1873–1875. - PubMed
-
- Frankel MS, Chapman AR. American Association for the Advancement of Sciences. 2000. Human Inheritable Genetic Modifications. Assessing Scientific, Ethical, Religious and Policy Issues.
-
- Davis BD. Germ-line therapy: evolutionary and moral considerations. Hum Gene Ther. 1992;3(4):361–363. - PubMed
-
- Neel JV. Germ-line gene therapy: another view. Hum Gene Ther. 1993;4(2):127–128. - PubMed
-
- Glover J. What Sort of People Should There Be? London: Penguin Books; 1984. pp. 45–47.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

