Natural and experimental hepatitis E virus genotype 3-infection in European wild boar is transmissible to domestic pigs
- PMID: 25421429
- PMCID: PMC4243386
- DOI: 10.1186/s13567-014-0121-8
Natural and experimental hepatitis E virus genotype 3-infection in European wild boar is transmissible to domestic pigs
Abstract
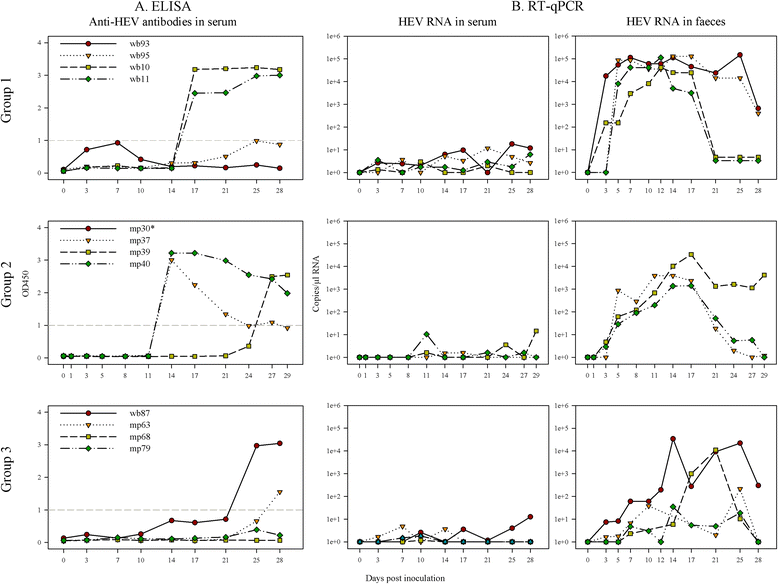
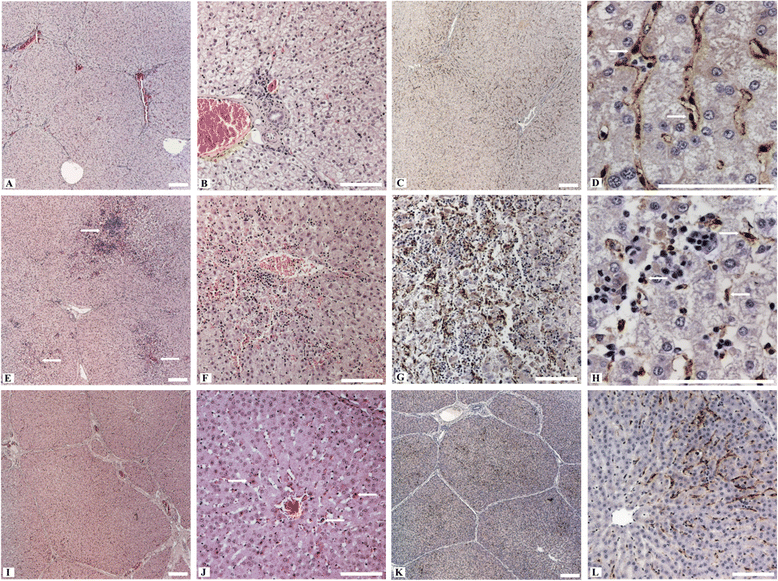
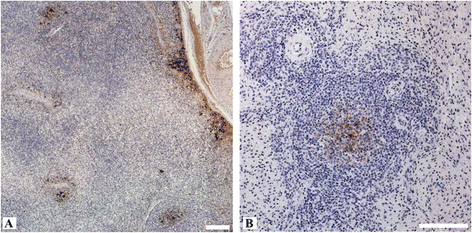
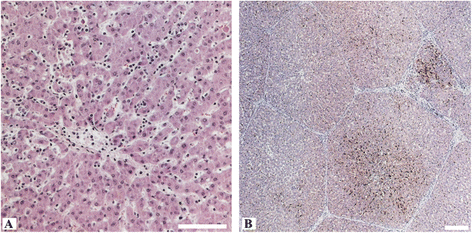
Hepatitis E virus (HEV) is the causative agent of acute hepatitis E in humans in developing countries, but sporadic and autochthonous cases do also occur in industrialised countries. In Europe, food-borne zoonotic transmission of genotype 3 (gt3) has been associated with domestic pig and wild boar. However, little is known about the course of HEV infection in European wild boar and their role in HEV transmission to domestic pigs. To investigate the transmissibility and pathogenesis of wild boar-derived HEVgt3, we inoculated four wild boar and four miniature pigs intravenously. Using quantitative real-time RT-PCR viral RNA was detected in serum, faeces and in liver, spleen and lymph nodes. The antibody response evolved after fourteen days post inoculation. Histopathological findings included mild to moderate lymphoplasmacytic hepatitis which was more prominent in wild boar than in miniature pigs. By immunohistochemical methods, viral antigens were detected mainly in Kupffer cells and liver sinusoidal endothelial cells, partially associated with hepatic lesions, but also in spleen and lymph nodes. While clinical symptoms were subtle and gross pathology was inconspicuous, increased liver enzyme levels in serum indicated hepatocellular injury. As the faecal-oral route is supposed to be the most likely transmission route, we included four contact animals to prove horizontal transmission. Interestingly, HEVgt3-infection was also detected in wild boar and miniature pigs kept in contact to intravenously inoculated wild boar. Given the high virus loads and long duration of viral shedding, wild boar has to be considered as an important HEV reservoir and transmission host in Europe.
Figures

References
-
- Emerson SU, Purcell RH. Fields Virology. 2. Philadelphia: Wolters Kluwer Health/Lippincott Williams and Wilkins; 2013. Hepatitis E virus; pp. 2242–2258.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

