Aberrant glycosylation promotes lung cancer metastasis through adhesion to galectins in the metastatic niche
- PMID: 25421439
- PMCID: PMC4367955
- DOI: 10.1158/2159-8290.CD-13-0760
Aberrant glycosylation promotes lung cancer metastasis through adhesion to galectins in the metastatic niche
Abstract
Metastasis is the leading cause of cancer-associated deaths. Although dissemination of tumor cells likely occurs early in tumorigenesis, the constituents of the microenvironment play essential rate-limiting roles in determining whether these cells will form clinically relevant tumors. Recent studies have uncovered many molecular factors that contribute to the establishment of a protumorigenic metastatic niche. Here, we demonstrate that galectin-3, whose expression has clinical associations with advanced malignancy and poor outcome, contributes to metastatic niche formation by binding to carbohydrates on metastatic cells. We show that galectin-3 is expressed early during tumorigenesis by both CD11b(+)Gr-1(+) and CD11b(+)Ly-6C(hi) leukocytes. Tumors mobilize these myeloid populations through secretion of soluble factors, including IL6. We find that metastatic cancer cells exhibit elevated presentation of the oncofetal galectin-3 carbohydrate ligand, the Thomsen-Friedenreich antigen, on their surfaces as a result of altered C2GnT2 and St6GalNAc4 glycosyltransferase activity that inhibits further glycosylation of this carbohydrate motif and promotes metastasis.
Significance: Although clinical observations of elevated serum galectin-3 levels and altered glycosylation have been associated with malignancy, we identify novel roles for glycosyltransferases in promoting adhesion to galectins in the metastatic niche. This identification of a cytokine-leukocyte-glycosylation axis in metastasis provides mechanistic explanations for clinical associations between malignancy and aberrant glycosylation.
©2014 American Association for Cancer Research.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures

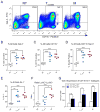

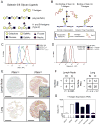
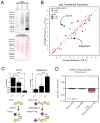
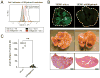

Comment in
-
Sweets for a bitter end: lung cancer cell-surface protein glycosylation mediates metastatic colonization.Cancer Discov. 2015 Feb;5(2):109-11. doi: 10.1158/2159-8290.CD-15-0013. Cancer Discov. 2015. PMID: 25656895 Free PMC article.
References
-
- Liu F-T, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41. - PubMed
-
- Almkvist J, Karlsson A. Galectins as inflammatory mediators. Glycoconj J. 2004;19:575–81. - PubMed
-
- Rabinovich GA, Toscano MA. Turning ‘sweet’ on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat Rev Immunol. 2009;9:338–52. - PubMed
-
- Iurisci I, Tinari N, Natoli C, Angelucci D, Cianchetti E, Iacobelli S. Concentrations of Galectin-3 in the Sera of Normal Controls and Cancer Patients. Clinical Cancer Research. 2000;6:1389–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

