Analysis of the phase 3 ESTABLISH trials of tedizolid versus linezolid in acute bacterial skin and skin structure infections
- PMID: 25421472
- PMCID: PMC4335893
- DOI: 10.1128/AAC.03688-14
Analysis of the phase 3 ESTABLISH trials of tedizolid versus linezolid in acute bacterial skin and skin structure infections
Abstract
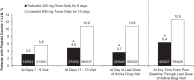
Tedizolid, a novel oxazolidinone with activity against a wide range of Gram-positive pathogens, was evaluated in two noninferiority phase 3 acute bacterial skin and skin structure infection trials. The data from individual trials showed its noninferior efficacy compared to that of linezolid and a favorable tolerability profile. To evaluate potential differences, the pooled data were analyzed. The patients received 200 mg of tedizolid once daily for 6 days or 600 mg of linezolid twice daily for 10 days. Efficacy was evaluated at 48 to 72 h (primary endpoint), on days 11 to 13 (end of therapy [EOT]), and 7 to 14 days after the EOT (posttherapy evaluation). Treatment-emergent adverse events and hematologic and clinical laboratory parameters were collected. The baseline characteristics were comparable between the treatment groups: 852/1,333 (64%) patients were from North America, and the majority of infections were caused by Staphylococcus aureus. Tedizolid was noninferior to linezolid (early clinical responses, 81.6% versus 79.4%, respectively). The early responses remained relatively consistent across various host/disease factors and severity measures. Nausea was the most frequently reported adverse event (tedizolid, 8.2%; linezolid, 12.2%; P=0.02), with onset occurring primarily during the first 6 days. Fewer tedizolid than linezolid patients had platelet counts of <150,000 cells/mm3 at the EOT (tedizolid, 4.9%; linezolid, 10.8%; P=0.0003) and during the postbaseline period through the last day of active drug visit (tedizolid, 6.4%; linezolid, 12.6%; P=0.0016). Efficacy was achieved with a 6-day once-daily course of therapy with the option of an intravenous/oral regimen, and fewer low platelet counts and gastrointestinal side effects were reported with tedizolid than with linezolid, all of which aligns well with antimicrobial stewardship principles. (These studies have been registered at ClinicalTrials.gov under registration no. NCT01170221 and NCT01421511.).
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

