First-in-humans study of the safety, tolerability, and pharmacokinetics of ACT-451840, a new chemical entity with antimalarial activity
- PMID: 25421475
- PMCID: PMC4335896
- DOI: 10.1128/AAC.04125-14
First-in-humans study of the safety, tolerability, and pharmacokinetics of ACT-451840, a new chemical entity with antimalarial activity
Abstract
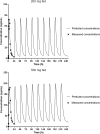
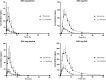
Emerging resistance to antimalarial agents raises the need for new drugs. ACT-451840 is a new compound with potent activity against sensitive and resistant Plasmodium falciparum strains. This was a first-in-humans single-ascending-dose study to investigate the safety, tolerability, and pharmacokinetics of ACT-451840 across doses of 10, 50, 200, and 500 mg in healthy male subjects. In the 200- and 500-mg dose groups, the effect of food was investigated, and antimalarial activity was assessed using an ex vivo bioassay with P. falciparum. No (serious) adverse events leading to discontinuation were reported. At the highest dose level, the peak drug concentration (Cmax) and the area under the plasma concentration-time curve from zero to infinity of ACT-451840 under fasted conditions reached 11.9 ng/ml and 100.6 ng·h/ml, respectively, and these were approximately 13-fold higher under fed conditions. Food did not affect the half-life (approximately 34 h) of the drug, while the Cmax was attained 2.0 and 3.5 h postdose under fasted and fed conditions, respectively. The plasma concentrations estimated by the bioassay were approximately 4-fold higher than those measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Several potentially active metabolites were also identified. ACT-451840 was well tolerated across all doses. Exposure to ACT-451840 significantly increased with food. The bioassay indicated the presence of circulating active metabolites. (This study has been registered at ClinicalTrials.gov under registration no. NCT02186002.).
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



References
-
- World Health Organization (WHO). 2008. Global malaria action plan, for a malaria-free world. World Health Organization, Geneva, Switzerland: http://www.rollbackmalaria.org/gmap/gmap.pdf.
-
- World Health Organization (WHO). 2012. WHO, world malaria report 2012. World Health Organization, Geneva, Switzerland: http://www.who.int/malaria/publications/world_malaria_report_2012/wmr201....
-
- Noor AM, Kinyoki DK, Mundia CW, Kabaria CW, Mutua JW, Alegana VA, Fall IS, Snow RW. 2014. The changing risk of Plasmodium falciparum malaria infection in Africa: 2000–10: a spatial and temporal analysis of transmission intensity. Lancet 383:1739–1747. doi:10.1016/S0140-6736(13)62566-0. - DOI - PMC - PubMed
-
- World Health Organization (WHO). 2012. Global malaria program: update on artemisinin resistance, April 2012. World Health Organization, Geneva, Switzerland: http://www.who.int/malaria/publications/atoz/arupdate042012.pdf?ua=1.
-
- World Health Organization (WHO). 2012. Emergency response to artemisinin resistance in the greater Mekong subregion: regional framework for action 2013–2015. World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/79940/1/9789241505321_eng.pdf?ua=1.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

