Anti-malarial activity of a polyherbal product (Nefang) during early and established Plasmodium infection in rodent models
- PMID: 25421605
- PMCID: PMC4251988
- DOI: 10.1186/1475-2875-13-456
Anti-malarial activity of a polyherbal product (Nefang) during early and established Plasmodium infection in rodent models
Abstract
Background: The emerging resistance of Plasmodium species to currently available anti-malarials remains a public health concern, hence the need for new effective, safe and affordable drugs. Natural products remain a reliable source of drugs. Nefang is a polyherbal anti-malarial of the Cameroonian folklore medicine with demonstrated in vitro antiplasmodial and antioxidant activities. It is composed of Mangifera indica (bark and leaf), Psidium guajava, Carica papaya, Cymbopogon citratus, Citrus sinensis, Ocimum gratissimum (leaves). This study aimed at investigating the suppressive, prophylactic and curative activities of Nefang in Plasmodium infected rodent models.
Methods: Systemic acute oral toxicity of Nefang aqueous and ethanol extracts was assessed in mice up to a dose of 5,000 mgkg(-1) body weight. BALB/c mice and Wistar rats were inoculated with Plasmodium chabaudi chabaudi and Plasmodium berghei, respectively, and treated with Nefang, the Mangifera indica bark/Psidium guajava combination and a Psidium guajava leaf aqueous extracts (75, 150, 300 and 600 mgkg(-1) bwt). Their schizonticidal activity was then evaluated using the Peter's 4-day suppressive test). The prophylactic and curative (Rane's Test) activity of Nefang was also evaluated by determining the parasitaemia, survival time, body weight and temperature in pre-treated rodents.
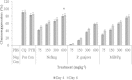
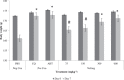
Results: Acute oral toxicity of the extract did not cause any observed adverse effects. Percent suppressions of parasitaemia at 600 mgkg(-1) bwt were as follows (P. berghei/P. chabaudi): Nefang - 82.9/86.3, Mangifera indica bark/Psidium guajava leaf combination extract - 79.5/81.2 and Psidium guajava leaf - 58.9/67.4. Nefang exhibited a prophylactic activity of 79.5% and its chemotherapeutic effects ranged from 61.2 - 86.1% with maximum effect observed at the highest experimental dose.
Conclusion: These results indicate that Nefang has excellent in vivo anti-malarial activities against P. berghei and P. chabaudi, upholding earlier in vitro antiplasmodial activities against multi-drug resistant P. falciparum parasites as well as its traditional use. Hence, Nefang represents a promising source of new anti-malarial agents.
Figures







Similar articles
-
Pharmacological evidence for the folk use of Nefang: antipyretic, anti-inflammatory and antinociceptive activities of its constituent plants.BMC Complement Altern Med. 2015 Jun 9;15:174. doi: 10.1186/s12906-015-0703-7. BMC Complement Altern Med. 2015. PMID: 26055261 Free PMC article.
-
In vitro antiplasmodial activities and synergistic combinations of differential solvent extracts of the polyherbal product, Nefang.Biomed Res Int. 2014;2014:835013. doi: 10.1155/2014/835013. Epub 2014 Apr 27. Biomed Res Int. 2014. PMID: 24877138 Free PMC article.
-
Antioxidant potential of a polyherbal antimalarial as an indicator of its therapeutic value.Adv Pharmacol Sci. 2013;2013:678458. doi: 10.1155/2013/678458. Epub 2013 Dec 19. Adv Pharmacol Sci. 2013. PMID: 24454347 Free PMC article.
-
Natural products as starting points for future anti-malarial therapies: going back to our roots?Malar J. 2011 Mar 15;10 Suppl 1(Suppl 1):S3. doi: 10.1186/1475-2875-10-S1-S3. Malar J. 2011. PMID: 21411014 Free PMC article. Review.
-
Antiplasmodial natural products: an update.Malar J. 2019 Dec 5;18(1):404. doi: 10.1186/s12936-019-3026-1. Malar J. 2019. PMID: 31805944 Free PMC article. Review.
Cited by
-
Indigenous medicinal plants used in folk medicine for malaria treatment in Kwara State, Nigeria: an ethnobotanical study.BMC Complement Med Ther. 2023 Sep 16;23(1):324. doi: 10.1186/s12906-023-04131-4. BMC Complement Med Ther. 2023. PMID: 37716985 Free PMC article.
-
Natural Products as Sources of Antimalarial Drugs: Ethnobotanical and Ethnopharmacological Studies.Scientifica (Cairo). 2020 May 9;2020:7076139. doi: 10.1155/2020/7076139. eCollection 2020. Scientifica (Cairo). 2020. PMID: 32455050 Free PMC article.
-
The Antiplasmodial Potential of Medicinal Plants Used in the Cameroonian Pharmacopoeia: An Updated Systematic Review and Meta-Analysis.Evid Based Complement Alternat Med. 2022 Oct 8;2022:4661753. doi: 10.1155/2022/4661753. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 36254175 Free PMC article. Review.
-
Hydroethanolic Extracts of Erigeron floribundus and Azadirachta indica Reduced Plasmodium berghei Parasitemia in Balb/c Mice.Evid Based Complement Alternat Med. 2018 Oct 21;2018:5156710. doi: 10.1155/2018/5156710. eCollection 2018. Evid Based Complement Alternat Med. 2018. PMID: 30420894 Free PMC article.
-
Anti-Malarial and Anti-Lipid Peroxidation Activities of Deferiprone-Resveratrol Hybrid in Plasmodium berghei-Infected Mice.Biology (Basel). 2021 Sep 14;10(9):911. doi: 10.3390/biology10090911. Biology (Basel). 2021. PMID: 34571788 Free PMC article.
References
-
- WHO: World Malaria Report 2013. World Health Organization. Geneva; http://www.who.int/malaria/publications/world_malaria_report_2013/en/
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

