EURAMOS-1, an international randomised study for osteosarcoma: results from pre-randomisation treatment
- PMID: 25421877
- PMCID: PMC4304379
- DOI: 10.1093/annonc/mdu526
EURAMOS-1, an international randomised study for osteosarcoma: results from pre-randomisation treatment
Abstract
Background: Four international study groups undertook a large study in resectable osteosarcoma, which included two randomised controlled trials, to determine the effect on survival of changing post-operative chemotherapy based on histological response.
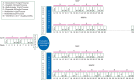
Patients and methods: Patients with resectable osteosarcoma aged ≤40 years were treated with the MAP regimen, comprising pre-operatively of two 5-week cycles of cisplatin 120 mg/m(2), doxorubicin 75 mg/m(2), methotrexate 12 g/m(2) × 2 (MAP) and post-operatively two further cycles of MAP and two cycles of just MA. Patients were randomised after surgery. Those with ≥10% viable tumour in the resected specimen received MAP or MAP with ifosfamide and etoposide. Those with <10% viable tumour were allocated to MAP or MAP followed by pegylated interferon. Longitudinal evaluation of quality of life was undertaken.
Results: Recruitment was completed to the largest osteosarcoma study to date in 75 months. Commencing March 2005, 2260 patients were registered from 326 centres across 17 countries. About 1334 of 2260 registered patients (59%) were randomised. Pre-operative chemotherapy was completed according to protocol in 94%. Grade 3-4 neutropenia affected 83% of cycles and 59% were complicated by infection. There were three (0.13%) deaths related to pre-operative chemotherapy. At definitive surgery, 50% of patients had at least 90% necrosis in the resected specimen.
Conclusions: New models of collaboration are required to successfully conduct trials to improve outcomes of patients with rare cancers; EURAMOS-1 demonstrates achievability. Considerable regulatory, financial and operational challenges must be overcome to develop similar studies in the future. The trial is registered as NCT00134030 and ISRCTN 67613327.
Keywords: international collaboration; osteosarcoma; randomised controlled trial; trial conduct.
© The Author 2014. Published by Oxford University Press on behalf of the European Society for Medical Oncology.
Figures
References
-
- Whelan J, McTiernan A, Cooper N, et al. Incidence and survival of malignant bone sarcomas in England 1979–2007. Int J Cancer. 2011;131:E508–E517. - PubMed
-
- Marina N, Bielack S, Whelan J, et al. International collaboration is feasible in trials for rare conditions: the EURAMOS experience. Cancer Treat Res. 2009;152:339–353. - PubMed
-
- Rosen G, Murphy ML, Huvos AG, et al. Chemotherapy, en bloc resection, and prosthetic bone replacement in the treatment of osteogenic sarcoma. Cancer. 1976;37:1–11. - PubMed
-
- Bacci G, Picci P, Ruggieri P, et al. Primary chemotherapy and delayed surgery (neoadjuvant chemotherapy) for osteosarcoma of the extremities. Cancer. 1990;65:2539–2553. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical