Euthanasia assessment in ebola virus infected nonhuman primates
- PMID: 25421892
- PMCID: PMC4246243
- DOI: 10.3390/v6114666
Euthanasia assessment in ebola virus infected nonhuman primates
Abstract
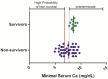
Multiple products are being developed for use against filoviral infections. Efficacy for these products will likely be demonstrated in nonhuman primate models of filoviral disease to satisfy licensure requirements under the Animal Rule, or to supplement human data. Typically, the endpoint for efficacy assessment will be survival following challenge; however, there exists no standardized approach for assessing the health or euthanasia criteria for filovirus-exposed nonhuman primates. Consideration of objective criteria is important to (a) ensure test subjects are euthanized without unnecessary distress; (b) enhance the likelihood that animals exhibiting mild or moderate signs of disease are not prematurely euthanized; (c) minimize the occurrence of spontaneous deaths and loss of end-stage samples; (d) enhance the reproducibility of experiments between different researchers; and (e) provide a defensible rationale for euthanasia decisions that withstands regulatory scrutiny. Historic records were compiled for 58 surviving and non-surviving monkeys exposed to Ebola virus at the US Army Medical Research Institute of Infectious Diseases. Clinical pathology parameters were statistically analyzed and those exhibiting predicative value for survival are reported. These findings may be useful for standardization of objective euthanasia assessments in rhesus monkeys exposed to Ebola virus and may serve as a useful approach for other standardization efforts.
Figures



References
-
- Geisbert T.W., Lee A.C., Robbins M., Geisbert J.B., Honko A.N., Sood V., Johnson J.C., de Jong S., Tavakoli I., Judge A., et al. Postexposure protection of non-human primates against a lethal Ebola virus challenge with RNA interference: A proof-of-concept study. Lancet. 2010;375:1896–1905. doi: 10.1016/S0140-6736(10)60357-1. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

