Shaping the intestinal brush border
- PMID: 25422372
- PMCID: PMC4242837
- DOI: 10.1083/jcb.201407015
Shaping the intestinal brush border
Abstract
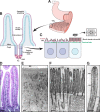
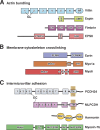
Epithelial cells from diverse tissues, including the enterocytes that line the intestinal tract, remodel their apical surface during differentiation to form a brush border: an array of actin-supported membrane protrusions known as microvilli that increases the functional capacity of the tissue. Although our understanding of how epithelial cells assemble, stabilize, and organize apical microvilli is still developing, investigations of the biochemical and physical underpinnings of these processes suggest that cells coordinate cytoskeletal remodeling, membrane-cytoskeleton cross-linking, and extracellular adhesion to shape the apical brush border domain.
© 2014 Crawley et al.
Figures


References
-
- Ahmed Z.M., Goodyear R., Riazuddin S., Lagziel A., Legan P.K., Behra M., Burgess S.M., Lilley K.S., Wilcox E.R., Riazuddin S., et al. . 2006. The tip-link antigen, a protein associated with the transduction complex of sensory hair cells, is protocadherin-15. J. Neurosci. 26:7022–7034 10.1523/JNEUROSCI.1163-06.2006 - DOI - PMC - PubMed
-
- Alagramam K.N., Goodyear R.J., Geng R., Furness D.N., van Aken A.F., Marcotti W., Kros C.J., and Richardson G.P.. 2011. Mutations in protocadherin 15 and cadherin 23 affect tip links and mechanotransduction in mammalian sensory hair cells. PLoS ONE. 6:e19183 10.1371/journal.pone.0019183 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

