Actin stress in cell reprogramming
- PMID: 25422450
- PMCID: PMC4267376
- DOI: 10.1073/pnas.1411683111
Actin stress in cell reprogramming
Abstract
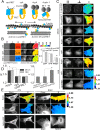
Cell mechanics plays a role in stem cell reprogramming and differentiation. To understand this process better, we created a genetically encoded optical probe, named actin-cpstFRET-actin (AcpA), to report forces in actin in living cells in real time. We showed that stemness was associated with increased force in actin. We reprogrammed HEK-293 cells into stem-like cells using no transcription factors but simply by softening the substrate. However, Madin-Darby canine kidney (MDCK) cell reprogramming required, in addition to a soft substrate, Harvey rat sarcoma viral oncogene homolog expression. Replating the stem-like cells on glass led to redifferentiation and reduced force in actin. The actin force probe was a FRET sensor, called cpstFRET (circularly permuted stretch sensitive FRET), flanked by g-actin subunits. The labeled actin expressed efficiently in HEK, MDCK, 3T3, and bovine aortic endothelial cells and in multiple stable cell lines created from those cells. The viability of the cell lines demonstrated that labeled actin did not significantly affect cell physiology. The labeled actin distribution was similar to that observed with GFP-tagged actin. We also examined the stress in the actin cross-linker actinin. Actinin force was not always correlated with actin force, emphasizing the need for addressing protein specificity when discussing forces. Because actin is a primary structural protein in animal cells, understanding its force distribution is central to understanding animal cell physiology and the many linked reactions such as stress-induced gene expression. This new probe permits measuring actin forces in a wide range of experiments on preparations ranging from isolated proteins to transgenic animals.
Keywords: actin; cell mechanics; force probe; reprogramming; stem cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

