SWOG S0221: a phase III trial comparing chemotherapy schedules in high-risk early-stage breast cancer
- PMID: 25422488
- PMCID: PMC4268253
- DOI: 10.1200/JCO.2014.56.3296
SWOG S0221: a phase III trial comparing chemotherapy schedules in high-risk early-stage breast cancer
Abstract
Purpose: To determine the optimal dose and schedule of anthracycline and taxane administration as adjuvant therapy for early-stage breast cancer.
Patients and methods: A 2 × 2 factorial design was used to test two hypotheses: (1) that a novel continuous schedule of doxorubicin-cyclophosphamide was superior to six cycles of doxorubicin-cyclophosphamide once every 2 weeks and (2) that paclitaxel once per week was superior to six cycles of paclitaxel once every 2 weeks in patients with node-positive or high-risk node-negative early-stage breast cancer. With 3,250 patients, a disease-free survival (DFS) hazard ratio of 0.82 for each randomization could be detected with 90% power with two-sided α = .05. Overall survival (OS) was a secondary outcome.
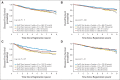
Results: Interim analyses crossed the futility boundaries for demonstrating superiority of both once-per-week regimens and once-every-2-weeks regimens. After a median follow-up of 6 years, a significant interaction developed between the two randomization factors (DFS P = .024; OS P = .010) in the 2,716 patients randomly assigned in the original design, which precluded interpretation of the two factors separately. Comparing all four arms showed a significant difference in OS (P = .040) but not in DFS (P = .11), with all treatments given once every 2 weeks associated with the highest OS. This difference in OS seemed confined to patients with hormone receptor-negative/human epidermal growth factor receptor 2 (HER2) -negative tumors (P = .067), with no differences seen with hormone receptor-positive/HER2-negative (P = .90) or HER2-positive tumors (P = .40).
Conclusion: Patients achieved a similar DFS with any of these regimens. Subset analysis suggests the hypothesis that once-every-2-weeks dosing may be best for patients with hormone receptor-negative/HER2-negative tumors.
© 2014 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures

References
-
- Joensuu H, Kellokumpu-Lehtinen PL, Huovinen R, et al. Adjuvant capecitabine, docetaxel, cyclophosphamide, and epirubicin for early breast cancer: Final analysis of the randomized FinXX trial. J Clin Oncol. 2011;30:11–18. - PubMed
-
- Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:1431–1439. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- N01 CA004919/CA/NCI NIH HHS/United States
- CA21076/CA/NCI NIH HHS/United States
- CA63848/CA/NCI NIH HHS/United States
- CA86780/CA/NCI NIH HHS/United States
- CA37981/CA/NCI NIH HHS/United States
- CA58416/CA/NCI NIH HHS/United States
- U10 CA027057/CA/NCI NIH HHS/United States
- N01 CA035176/CA/NCI NIH HHS/United States
- CA77597/CA/NCI NIH HHS/United States
- CA35128/CA/NCI NIH HHS/United States
- CA35261/CA/NCI NIH HHS/United States
- 015469/PHS HHS/United States
- U10 CA004919/CA/NCI NIH HHS/United States
- N01 CA035431/CA/NCI NIH HHS/United States
- CA22433/CA/NCI NIH HHS/United States
- U10 CA045560/CA/NCI NIH HHS/United States
- CA12644/CA/NCI NIH HHS/United States
- U10 CA035128/CA/NCI NIH HHS/United States
- CA20319/CA/NCI NIH HHS/United States
- U10 CA063845/CA/NCI NIH HHS/United States
- 021039/PHS HHS/United States
- U10 CA128567/CA/NCI NIH HHS/United States
- N01 CA032102/CA/NCI NIH HHS/United States
- N01 CA013612/CA/NCI NIH HHS/United States
- U10 CA077202/CA/NCI NIH HHS/United States
- U10 CA045808/CA/NCI NIH HHS/United States
- U10 CA021115/CA/NCI NIH HHS/United States
- U10 CA013612/CA/NCI NIH HHS/United States
- U10 CA180835/CA/NCI NIH HHS/United States
- CA58658/CA/NCI NIH HHS/United States
- CA68183/CA/NCI NIH HHS/United States
- CA35281/CA/NCI NIH HHS/United States
- N01 CA045807/CA/NCI NIH HHS/United States
- CA128567/CA/NCI NIH HHS/United States
- CCSRI15469/PHS HHS/United States
- CA63845/CA/NCI NIH HHS/United States
- U10 CA077597/CA/NCI NIH HHS/United States
- U10 CA014028/CA/NCI NIH HHS/United States
- N01 CA035119/CA/NCI NIH HHS/United States
- CA45808/CA/NCI NIH HHS/United States
- N01 CA046441/CA/NCI NIH HHS/United States
- U10 CA063848/CA/NCI NIH HHS/United States
- CA35262/CA/NCI NIH HHS/United States
- U10 CA021076/CA/NCI NIH HHS/United States
- CA14028/CA/NCI NIH HHS/United States
- CA58882/CA/NCI NIH HHS/United States
- CA45377/CA/NCI NIH HHS/United States
- U10 CA074647/CA/NCI NIH HHS/United States
- U10 CA035281/CA/NCI NIH HHS/United States
- CA11083/CA/NCI NIH HHS/United States
- CA58861/CA/NCI NIH HHS/United States
- CA35090/CA/NCI NIH HHS/United States
- CA76132/CA/NCI NIH HHS/United States
- N01 CA063844/CA/NCI NIH HHS/United States
- CA46282/CA/NCI NIH HHS/United States
- U10 CA035261/CA/NCI NIH HHS/United States
- U10 CA035178/CA/NCI NIH HHS/United States
- U10 CA045461/CA/NCI NIH HHS/United States
- CA76447/CA/NCI NIH HHS/United States
- U10 CA045450/CA/NCI NIH HHS/United States
- U10 CA032102/CA/NCI NIH HHS/United States
- U10 CA046282/CA/NCI NIH HHS/United States
- CA45450/CA/NCI NIH HHS/United States
- U10 CA035262/CA/NCI NIH HHS/United States
- CA46368/CA/NCI NIH HHS/United States
- CA67663/CA/NCI NIH HHS/United States
- N01 CA035178/CA/NCI NIH HHS/United States
- N01 CA038926/CA/NCI NIH HHS/United States
- CA45461/CA/NCI NIH HHS/United States
- U10 CA067575/CA/NCI NIH HHS/United States
- N01 CA027057/CA/NCI NIH HHS/United States
- CA77202/CA/NCI NIH HHS/United States
- U10 CA046441/CA/NCI NIH HHS/United States
- U10 CA045377/CA/NCI NIH HHS/United States
- U10 CA058882/CA/NCI NIH HHS/United States
- CA74647/CA/NCI NIH HHS/United States
- U10 CA020319/CA/NCI NIH HHS/United States
- U10 CA038926/CA/NCI NIH HHS/United States
- U10 CA086780/CA/NCI NIH HHS/United States
- U10 CA042777/CA/NCI NIH HHS/United States
- CA25224/CA/NCI NIH HHS/United States
- U10 CA035431/CA/NCI NIH HHS/United States
- U10 CA035119/CA/NCI NIH HHS/United States
- U10 CA095860/CA/NCI NIH HHS/United States
- CA42777/CA/NCI NIH HHS/United States
- U10 CA011083/CA/NCI NIH HHS/United States
- CA52654/CA/NCI NIH HHS/United States
- U10 CA046368/CA/NCI NIH HHS/United States
- N01 CA067575/CA/NCI NIH HHS/United States
- U10 CA067663/CA/NCI NIH HHS/United States
- U10 CA052654/CA/NCI NIH HHS/United States
- CA21115/CA/NCI NIH HHS/United States
- U10 CA025224/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- U10 CA180819/CA/NCI NIH HHS/United States
- U10 CA035176/CA/NCI NIH HHS/United States
- U10 CA035090/CA/NCI NIH HHS/United States
- U10 CA063844/CA/NCI NIH HHS/United States
- CA95860/CA/NCI NIH HHS/United States
- U10 CA058861/CA/NCI NIH HHS/United States
- U10 CA045807/CA/NCI NIH HHS/United States
- N01 CA045560/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

