Early non-steady-state population pharmacokinetics of oral cyclosporine in renal transplant recipients
- PMID: 25422583
- PMCID: PMC4232039
- DOI: 10.2147/DDDT.S70595
Early non-steady-state population pharmacokinetics of oral cyclosporine in renal transplant recipients
Abstract
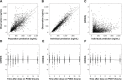
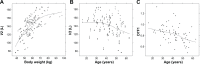
This study aimed to evaluate the change in the pharmacokinetics (PK) of cyclosporine in the non-steady-state period in the first week after renal transplantation; the factors influencing this change, including genetic variability; and the time point concentration that correlated best with drug exposure. Data were obtained from 69 patients, and PK studies were conducted on postoperative days (PODs) 2, 3, and 7. Samples were taken pre-dose and at 1, 2, 3, 4, 6, 8, and 12 hours after drug administration. MDR1, CYP3A4, and CYP3A5 were genotyped. A population PK analysis and correlational analysis between the concentration at each time point and the area under the time-concentration curve were performed. A two-compartment model with first-order absorption was chosen. The rate and extent of drug absorption showed a significant increase on POD3, followed by a slight decrease on POD7. Until POD3, 8 hours post-dose was the single time point concentration that correlated best with drug exposure and 3 hours was the best time point on POD7. In both analyses, the MDR1 genotype showed potential as a factor influencing PK change. We conclude that oral administration of cyclosporine and dose adjustment based on a single concentration measurement might result in unexpected drug exposure during this early posttransplantation period.
Keywords: cytochrome P450; multidrug resistance 1 (MDR1) gene; renal transplantation.
Figures

Similar articles
-
CYP3A7, CYP3A5, CYP3A4, and ABCB1 genetic polymorphisms, cyclosporine concentration, and dose requirement in transplant recipients.Ther Drug Monit. 2008 Dec;30(6):689-99. doi: 10.1097/FTD.0b013e31818a2a60. Ther Drug Monit. 2008. PMID: 18978522
-
[Pharmacogenetic analysis of the absorption kinetics of cyclosporine in a population of Spanish cardiac transplant patients].Farm Hosp. 2009 Nov-Dec;33(6):324-9. Farm Hosp. 2009. PMID: 20038391 Spanish.
-
CYP3A5 and MDR1 genetic polymorphisms and cyclosporine pharmacokinetics after renal transplantation.Clin Pharmacol Ther. 2004 May;75(5):422-33. doi: 10.1016/j.clpt.2004.01.009. Clin Pharmacol Ther. 2004. PMID: 15116055
-
Impact of CYP3A4 and MDR1 gene (G2677T) polymorphisms on dose requirement of the cyclosporine in renal transplant Egyptian recipients.Mol Biol Rep. 2015 Jan;42(1):105-17. doi: 10.1007/s11033-014-3747-8. Epub 2014 Sep 21. Mol Biol Rep. 2015. PMID: 25240575
-
CYP3A5 polymorphism effect on cyclosporine pharmacokinetics in living donor renal transplant recipients: analysis by population pharmacokinetics.Ann Pharmacother. 2012 Sep;46(9):1141-51. doi: 10.1345/aph.1R004. Epub 2012 Sep 4. Ann Pharmacother. 2012. PMID: 22947591
Cited by
-
Pharmacogenetics and drug-induced nephrotoxicity in renal transplant recipients.Bioimpacts. 2015;5(1):45-54. doi: 10.15171/bi.2015.12. Epub 2015 Feb 21. Bioimpacts. 2015. PMID: 25901296 Free PMC article. Review.
-
External evaluation of population pharmacokinetic models for ciclosporin in adult renal transplant recipients.Br J Clin Pharmacol. 2018 Jan;84(1):153-171. doi: 10.1111/bcp.13431. Epub 2017 Nov 3. Br J Clin Pharmacol. 2018. PMID: 28891596 Free PMC article.
References
-
- Utecht KN, Hiles JJ, Kolesar J. Effects of genetic polymorphisms on the pharmacokinetics of calcineurin inhibitors. Am J Health Syst Pharm. 2006;63(23):2340–2348. - PubMed
-
- Dunn CJ, Wagstaff AJ, Perry CM, Plosker GL, Goa KL. Cyclosporin: an updated review of the pharmacokinetic properties, clinical efficacy and tolerability of a microemulsion-based formulation (neoral)1 in organ transplantation. Drugs. 2001;61(13):1957–2016. - PubMed
-
- International Neoral Renal Transplantation Study Group Cyclosporine microemulsion (Neoral) absorption profiling and sparse-sample predictors during the first 3 months after renal transplantation. Am J Transplant. 2002;2(2):148–156. - PubMed
-
- Mahalati K, Belitsky P, Sketris I, West K, Panek R. Neoral monitoring by simplified sparse sampling area under the concentration-time curve: its relationship to acute rejection and cyclosporine nephrotoxicity early after kidney transplantation. Transplantation. 1999;68(1):55–62. - PubMed
-
- Canadian Neoral Renal Transplantation Study Group Absorption profiling of cyclosporine microemulsion (neoral) during the first 2 weeks after renal transplantation. Transplantation. 2001;72(6):1024–1032. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

