Inhibition of cardiac Kv1.5 potassium current by the anesthetic midazolam: mode of action
- PMID: 25422586
- PMCID: PMC4232042
- DOI: 10.2147/DDDT.S70461
Inhibition of cardiac Kv1.5 potassium current by the anesthetic midazolam: mode of action
Abstract
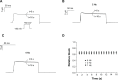
Midazolam is a short-acting benzodiazepine that is widely used in anesthesia. Despite its widespread clinical use, detailed information about cardiac side effects of midazolam is largely lacking. Using the double-electrode voltage clamp technique, we studied pharmacological effects of midazolam on heterologously expressed Kv1.5 channels underlying atrial repolarizing current I(Kur). Midazolam dose-dependently inhibited Kv1.5 current, yielding an IC50 of 17 μM in an HEK cell line and an IC50 of 104 μM in Xenopus oocytes. We further showed that midazolam did not affect the half-maximal activation voltage of Kv1.5 channels. However, a small negative shift of the inactivation curve could be observed. Midazolam acted as a typical open-channel inhibitor with rapid onset of block and without frequency dependence of block. Taken together, midazolam is an open channel inhibitor of cardiac Kv1.5 channels. These data add to the current understanding of the pharmacological profile of midazolam.
Keywords: anesthetics; pharmacology; potassium channels.
Figures



References
-
- Thummel KE, O’Shea D, Paine MF, et al. Oral first-pass elimination of midazolam involves both gastrointestinal and hepatic CYP3A-mediated metabolism. Clin Pharmacol Ther. 1996;59(5):491–502. - PubMed
-
- Nordt SP, Clark RF. Midazolam: a review of therapeutic uses and toxicity. J Emerg Med. 1997;15(3):357–365. - PubMed
-
- Nonaka A, Kashimoto S, Imamura M, Furuya A, Kumazawa T. Mechanism of the negative inotropic effect of midazolam and diazepam in cultured foetal mouse cardiac myocytes. Eur J Anaesthesiol. 1997;14(5):481–487. - PubMed
-
- Morani G, Bergamini C, Angheben C, et al. General anaesthesia for external electrical cardioversion of atrial fibrillation: experience of an exclusively cardiological procedural management. Europace. 2010;12(11):1558–1563. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

